Treble PCR method for simultaneously detecting mycoplasma hyopneumoniae, porcine pasteurella multocida and haemophilus parasuis
A technology for Mycoplasma hyopneumoniae and Haemophilus suis, which is applied in the field of multiplex PCR detection, can solve the problems of limited research, time-consuming and labor-intensive accuracy, harsh culture conditions, etc., and achieves the effects of strong specificity, rapid detection, and rapid specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Optimization of annealing temperature of triple PCR method for detecting Mycoplasma hyopneumoniae, Pasteurella multocida and Haemophilus parasuis
[0025] 1 sample preparation
[0026] Place the pig lung tissue sample sent from the pig farm in the mycoplasma liquid medium, culture for 24 hours and then take the bacterial liquid as a DNA sample for extracting Mycoplasma hyopneumoniae; the pig lung tissue sample sent from the pig farm is streaked on the TSA medium After culturing at 37°C for 24 hours, a single colony was picked and inoculated into TSB medium. After 12 hours, the bacterial solution was taken as a DNA sample for extracting Pasteurella multocida and Haemophilus parasuis.
[0027] 2 Extraction of bacterial DNA
[0028] The present invention adopts a conventional DNA extraction method, and the steps are as follows:
[0029] 2.1 Suspend the above bacterial liquid in 1.5ml lysozyme solution (0.15mol / l NaCl, 0.1mol / l NaCl 2 EDTA, 15mg / ml lysozyme, pH=8);
[0030]...
Embodiment 2
[0038] Example 2 The specificity of the triple PCR method system of Mycoplasma hyopneumoniae, Pasteurella multocida and Haemophilus parasuis
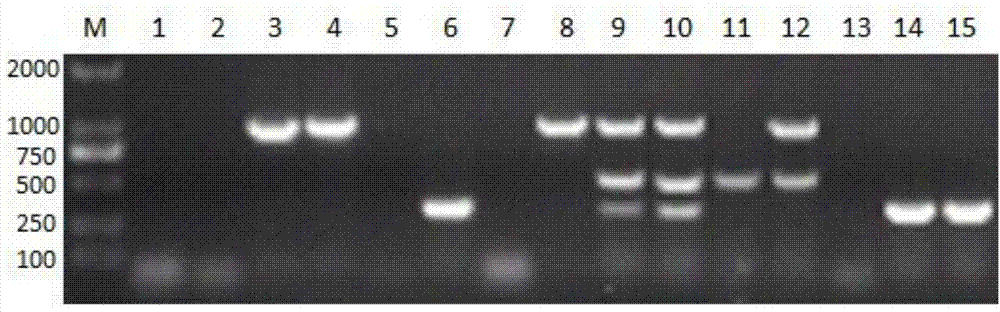
[0039] Specificity is another key factor of the PCR reaction system. In order to ensure the specificity of the established triple PCR system, the present invention uses Escherichia coli, Salmonella, Staphylococcus aureus, Streptococcus type 2 etc. as controls to verify the specificity of the system, and the system can only amplify Mycoplasma hyopneumoniae , The target gene fragments of Pasteurella multocida in pigs, but the control group cannot amplify the fragments as qualified for specificity.
[0040] 1 sample preparation
[0041] First, streak the Salmonella and Escherichia coli stored in the laboratory on the LB medium. After culturing overnight at 37°C, pick a single colony and inoculate it in 2mL LB liquid medium. After 12 hours, take the Salmonella and Escherichia coli as a control PCR template; Streak the Haemophilus parasuis and St...
Embodiment 3 3
[0048] Example 3 Triple PCR detection of Pasteurella multocida, Mycoplasma hyopneumoniae and Haemophilus parasuis in actual samples
[0049] 1 sample preparation
[0050] Choose TSB medium suitable for Pasteurella multocida, Mycoplasma hyopneumoniae, and Haemophilus parasuis, collect tissue samples from the field, and directly inoculate them in TSB liquid medium for 12 hours after aseptic operation. Extract the DNA sample of the pig tested pathogen as a template for triple PCR detection.
[0051] 2 Extraction of bacterial DNA
[0052] The same as in Example 1.
[0053] 3 Triple PCR amplification reaction
[0054] The triple PCR reaction system is as follows: cDNA template 1μl, 3 pairs of upstream and downstream primers each 0.5μl, PCR Mix 12.5μl, ultrapure water supplemented to 50μl. PCR reaction conditions: 95°C pre-denaturation for 5 minutes, enter 95°C 60s, 58°C 1min and 72°C 1min cycle, cycle 35 times, and finally extend at 72°C for 5 minutes. After PCR, take 8μl of product for aga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com