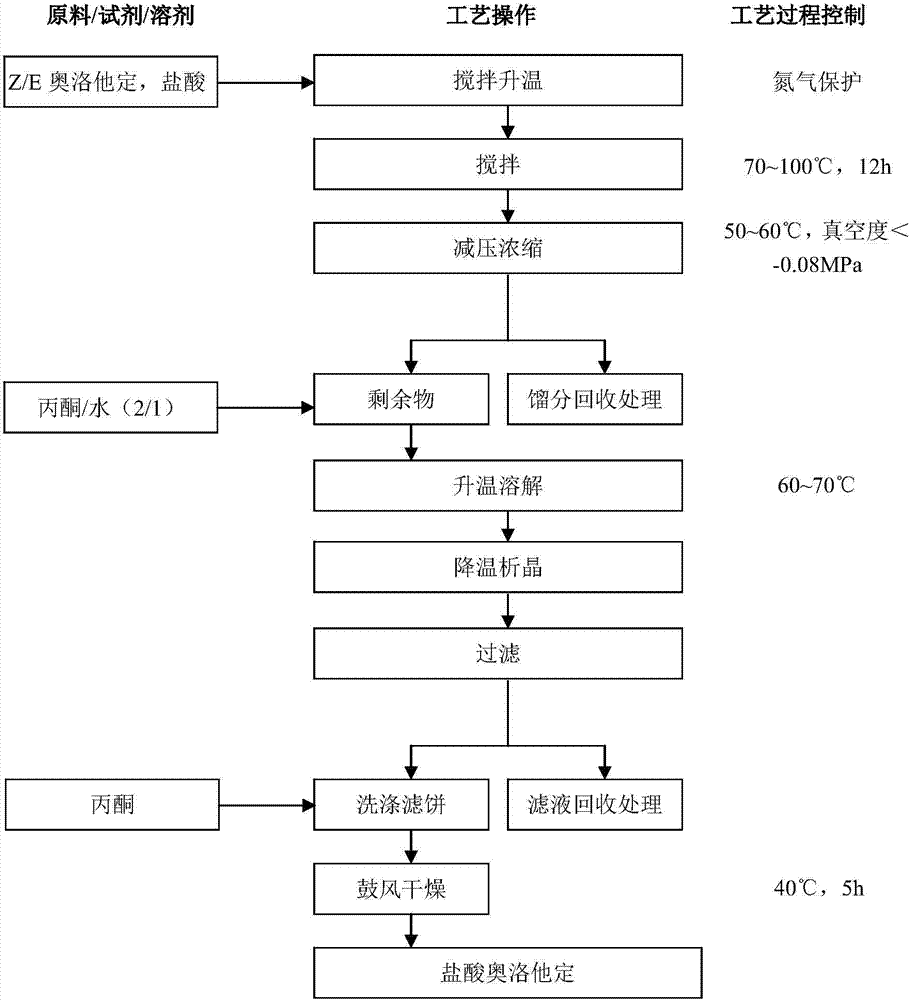
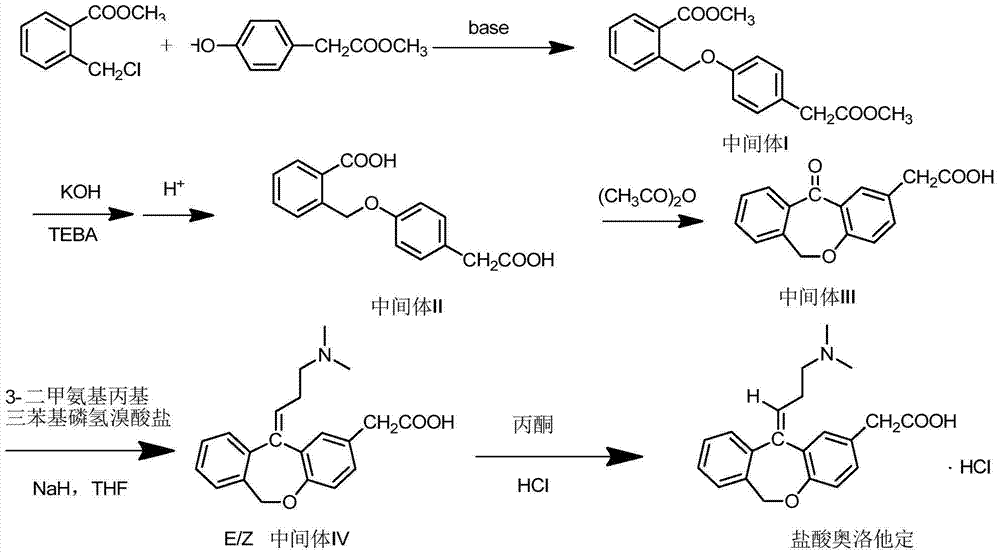
Method for preparing olopatadine hydrochloride
A technology of olopatadine hydrochloride and hydrochloric acid, which is applied in the field of preparation of olopatadine hydrochloride, can solve the problems of low yield, waste phosphoric acid, and unsuitability for industrial production, and achieve high yield, high total yield, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Step 1. Preparation of Intermediate I
[0036] Methyl 2-chloromethylbenzoate (100g, 0.54mol) and methyl p-hydroxyphenylacetate (90g, 0.54mol) were dissolved in 1L of ethanol, and 1.08mol of acid-binding agent (see the table below for dosage) was added, heated to reflux, according to TLC (petroleum ether: ethyl acetate = 5:1) monitored the reaction end point, cooled to room temperature, vacuum filtered, the filter cake was washed with ethanol (200mL*2), and the filtrate was concentrated under reduced pressure to obtain a yellow oil, which was the intermediate I. Wherein, acid-binding agent dosage and test result are shown in the following table:
[0037]
[0038] Step 2. Preparation of Intermediate II
[0039] Intermediate I (160g, 0.51mol) and 3.2g TEBA were dissolved in ethanol, stirred, potassium hydroxide (60g, 1.07mol) was added, heated to reflux for 3h, ethanol was distilled off, and the residue was dissolved in 1L of water. Acidify with concentrated HCl to p...
Embodiment 2
[0051] Step 1. Preparation of Intermediate I
[0052] Methyl 2-chloromethylbenzoate (2kg, 10.8mol) and methyl p-hydroxyphenylacetate (1.8kg, 10.8mol) were dissolved in 20L ethanol, and potassium carbonate (3kg, 21.6mol) was added, heated to reflux for 24h, cooled to Suction filtration under reduced pressure at room temperature, the filter cake was washed with ethanol (4L*2), and the filtrate was concentrated under reduced pressure to obtain 3.26kg of intermediate I as a yellow oil, with a yield of 95.6%.
[0053] Step 2. Preparation of Intermediate II
[0054] Dissolve intermediate I (3.2kg, 10.2mol) and 34g TEBA in ethanol, stir, add potassium hydroxide (1.2kg, 21.4mol), heat to reflux for 3h, distill off ethanol, and dissolve the residue with 20L of water. Acidify with concentrated HCl to pH = 1-2 to generate a large amount of light yellow solid, filter with suction, wash the filter cake with water until neutral, and dry to obtain 2.42kg of light yellow solid, which is Inte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com