Drug composition of oral rehydration salt and preparation method of drug composition
A technology of composition and rehydration salt, which is applied in the field of medicine and can solve unsatisfactory problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
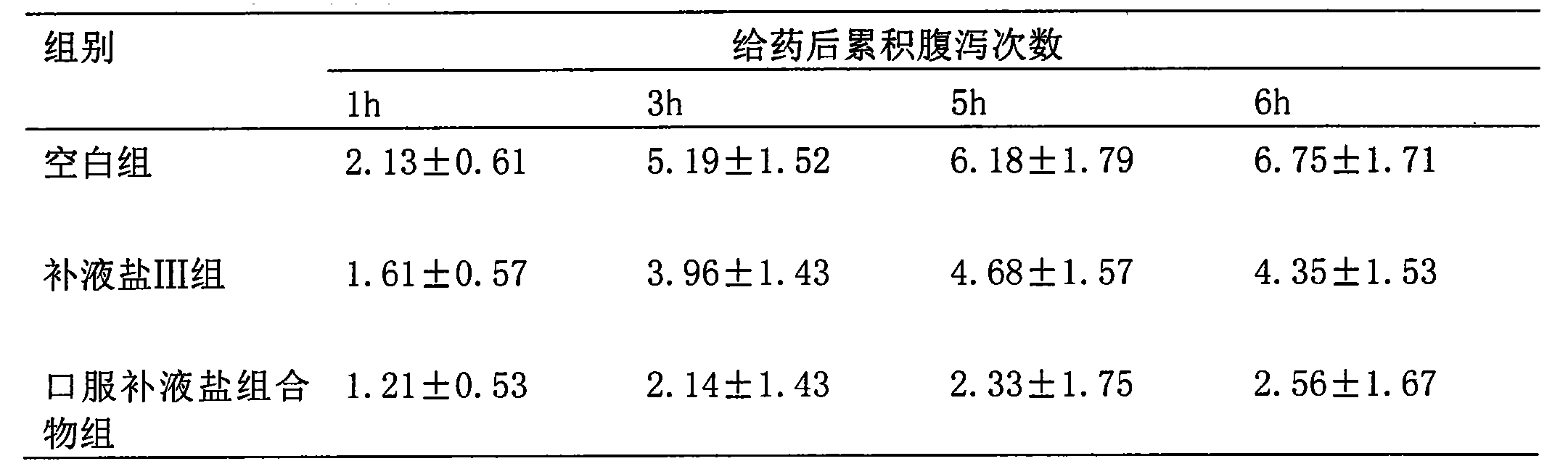
[0034] Effect of the oral rehydration salt composition of the present invention on castor oil-induced diarrhea in mice. 30 mice were randomly divided into 3 groups, respectively: blank group, rehydration salt III group, oral rehydration salt composition group of the present invention, the dosage was 1g / kg, 10 mice in each group, and castor oil 0.1 After 1 ml / 10g, the drug was given by intragastric administration, and then the mice were individually placed in a mouse cage with absorbent paper, and the paper was changed every 1 hour. Diarrhea frequency (loose stool points).
[0035] Table 1: Effect of oral rehydration salt composition of the present invention on castor oil-induced diarrhea in mice
[0036]
[0037] The results show that the oral rehydration salt composition of the present invention can obviously resist castor oil-induced diarrhea in mice.
Embodiment 2
[0039] 1) Prescription (1)
[0040]
[0041] 2) Prescription (1) Preparation process:
[0042] ① Raw material pretreatment:
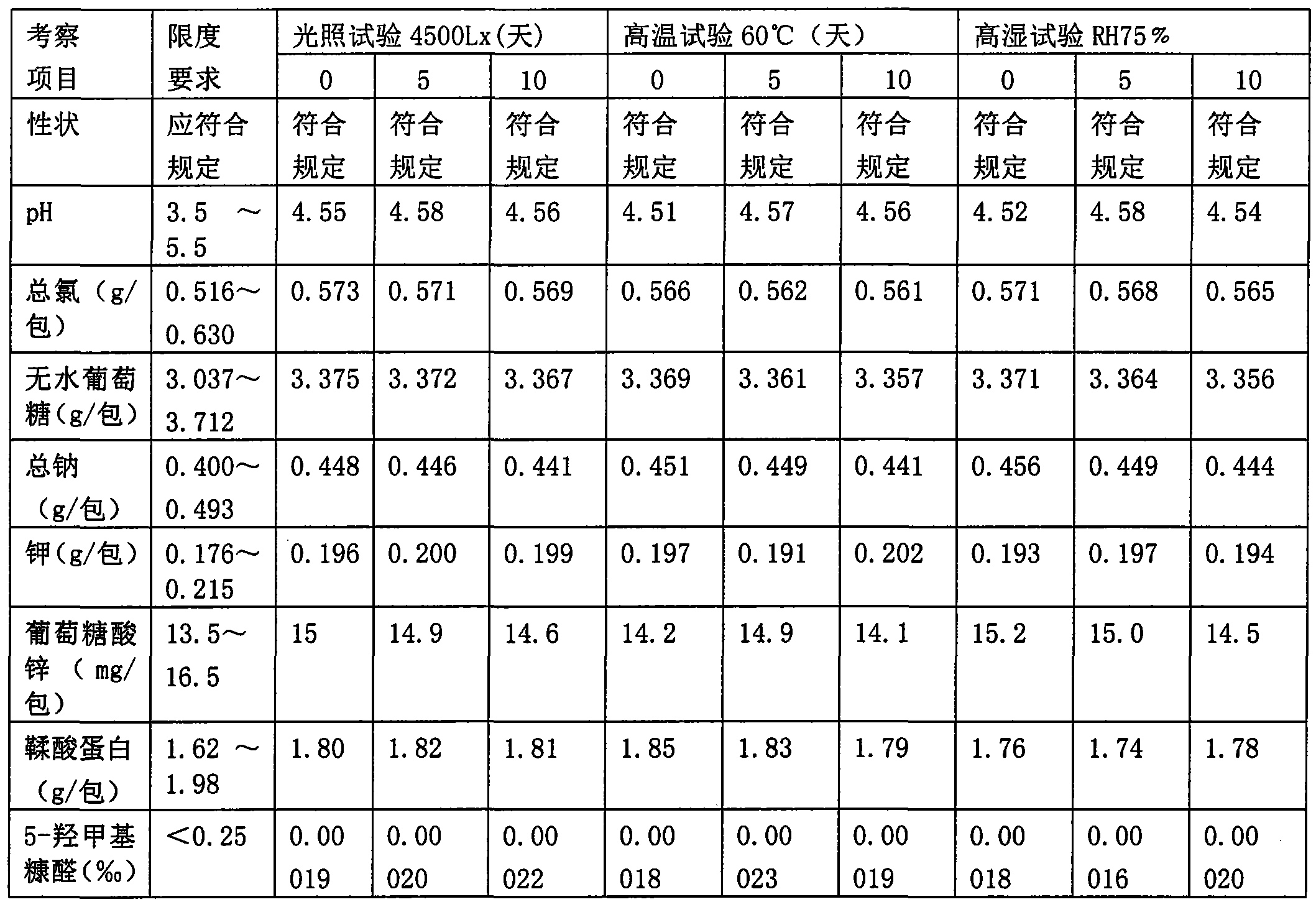
[0043] Sodium chloride, potassium chloride, sodium citrate, zinc gluconate, protein tannin, anhydrous glucose, and sucralose are crushed through an 80-mesh sieve, carmine pigment is passed through an 80-mesh sieve, strawberry essence is passed through a 60-mesh sieve, Anhydrous glucose is passed through a 40-mesh sieve for later use.
[0044] ②Mix:
[0045] Mix 25g of sucralose with 25g of strawberry essence for 2 minutes to obtain mixture A;
[0046] Mix 1.5 g of carmine and 1.5 g of zinc gluconate for 1 minute, add 3 g of zinc gluconate and mix for 2 minutes, add 6 g of zinc gluconate and mix for 2 minutes, add the remaining zinc gluconate and mix for 2 minutes to obtain mixture B;
[0047] Add mixture A and mix for 2 minutes, add sodium citrate and mix for 2 minutes, then add anhydrous citric acid, potassium chloride, and sodium chloride and m...
Embodiment 3
[0059] 1) Prescription (2)
[0060]
[0061] 2) Prescription (2) Preparation process:
[0062] ① Raw material pretreatment:
[0063]Sodium chloride, potassium chloride, sodium citrate, zinc gluconate, protein tannin, anhydrous glucose, and sucralose are crushed through an 80-mesh sieve, carmine pigment is passed through an 80-mesh sieve, strawberry essence is passed through a 60-mesh sieve, Anhydrous glucose is passed through a 40-mesh sieve for later use.
[0064] ②Mix:
[0065] Mix 25g of sucralose with 25g of strawberry essence for 2 minutes to obtain mixture A;
[0066] Mix 1.5 g of carmine and 1.5 g of zinc gluconate for 1 minute, add 3 g of zinc gluconate and mix for 2 minutes, add 6 g of zinc gluconate and mix for 2 minutes, add the remaining zinc gluconate and mix for 2 minutes to obtain mixture B;
[0067] Add mixture A and mix for 2 minutes, add sodium citrate and mix for 2 minutes, then add anhydrous citric acid, potassium chloride, and sodium chloride and mi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com