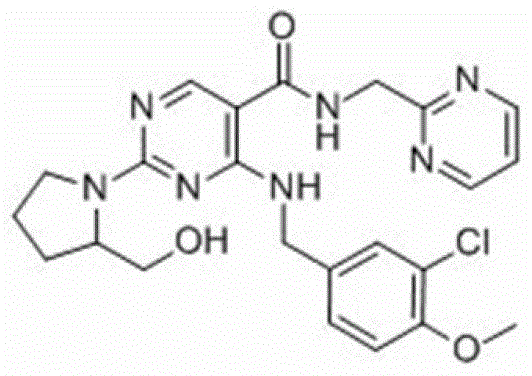
Avanafil effervescent dry suspension and preparation method thereof
A technology of avanafil and dry suspension, which is applied in the direction of medical preparations of non-active ingredients, pharmaceutical formulas, inorganic non-active ingredients, etc., and can solve the problem of not significantly improving the oral bioavailability of insoluble drugs and increasing the insoluble Problems such as drug solubility, large production safety hazards, etc., to achieve the effect of changing pharmacokinetic characteristics, ensuring a highly dispersed state, and fast onset of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0083] (2) Preparation method of avanafil effervescent dry suspension
[0084] method 1:
[0085] The present invention also provides a kind of preparation method of described avanafil effervescent dry suspension, described method comprises:
[0086]1) Fully dry the avanafil, the effervescent disintegrant, the filler, the suspending agent, and optional other pharmaceutical excipients respectively, and pulverize them to 100 mesh Particle size below the sieve; and
[0087] 2) Thoroughly mix the avanafil, effervescent disintegrating agent, filler, suspending agent, and optional other pharmaceutical excipients obtained in step 1), so as to obtain the avanafil effervescent dry mix Suspension.
[0088] Method 2:
[0089] The present invention also provides a kind of preparation method of described avanafil effervescent dry suspension, described method comprises:
[0090] a) Fully dry the avanafil, the effervescent disintegrant, the filler, the suspending agent, and optional oth...
Embodiment 1-3
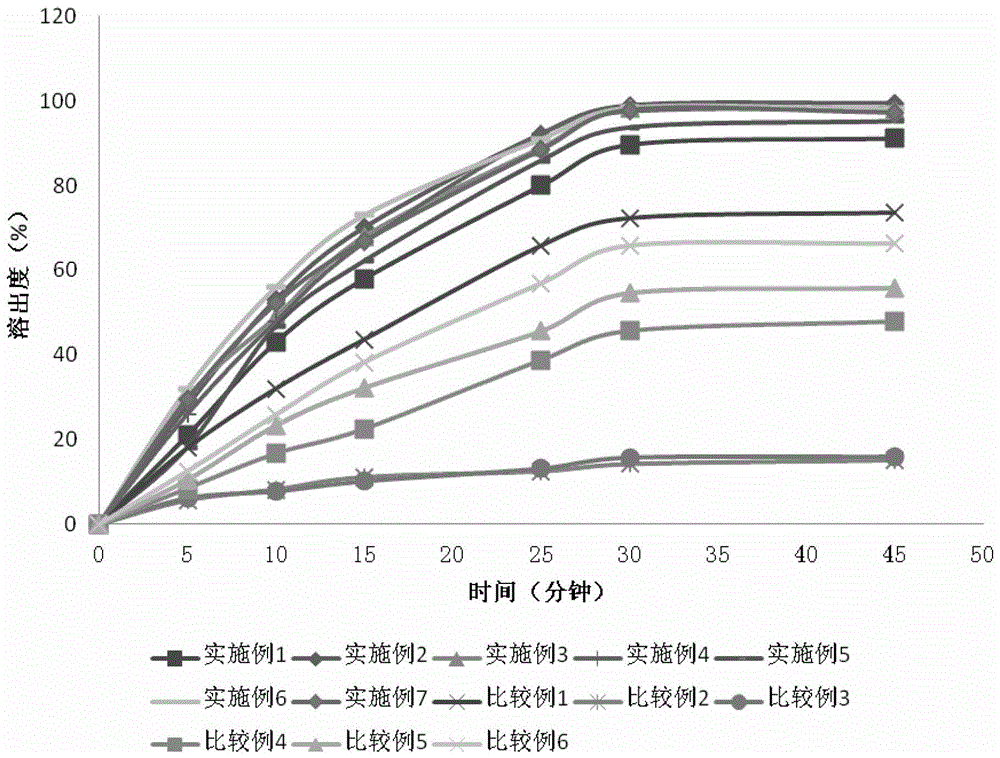
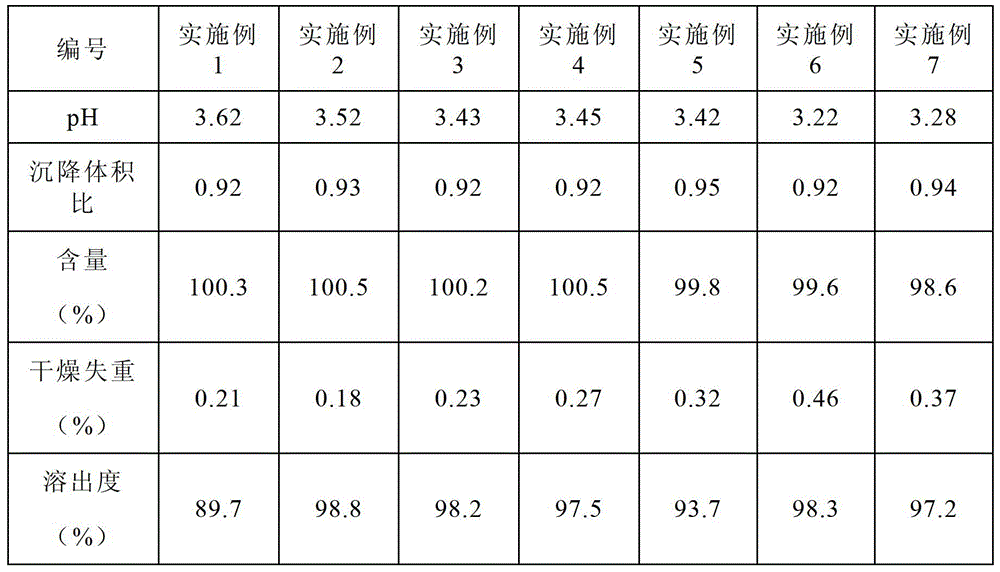
[0115] See Table 1 for the single dose formulations of Examples 1-3.
[0116] Table 1
[0117] raw material
Embodiment 1
[0118] Example 1 Preparation process: The raw and auxiliary materials were dried and crushed through a 100-mesh sieve, weighed according to the above prescription ratio, and the active ingredient avanafil was mixed with fillers, suspending agents, effervescent disintegrants, and flavoring agents (i.e., sweeteners). Flavor and essence, the same below) and colorant are mixed evenly, and 5% (w / v) povidone k30 absolute ethanol solution is used as a binder to make granules. After the granules are dry, add glidant and mix well. Packed in single doses.
[0119] The preparation process of Example 2: the raw and auxiliary materials were dried and crushed through a 100-mesh sieve, weighed according to the above-mentioned prescription ratio, and the active ingredient avanafil was evenly mixed with the filler, suspending agent, and effervescent disintegrating agent, and then added the correction agent Flavoring agent, glidant and coloring agent are thoroughly mixed and uniformly formed in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com