A kind of c-peptide monoclonal antibody cross-linked magnetic particle and its preparation method and detection kit including it
A monoclonal antibody and detection kit technology, applied in the field of in vitro diagnostic medical testing, can solve problems such as insufficient sensitivity, different BSA specificities, and non-specific binding.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] Correspondingly, the present invention also provides a method for preparing the above-mentioned C-peptide monoclonal antibody cross-linked magnetic particles, the method comprising the following steps:
[0060] a) cross-linking the C-peptide monoclonal antibody to the magnetic particles; and
[0061] b) Pretreating the C-peptide monoclonal antibody cross-linked magnetic particles obtained in step a) with a non-ionic surfactant.
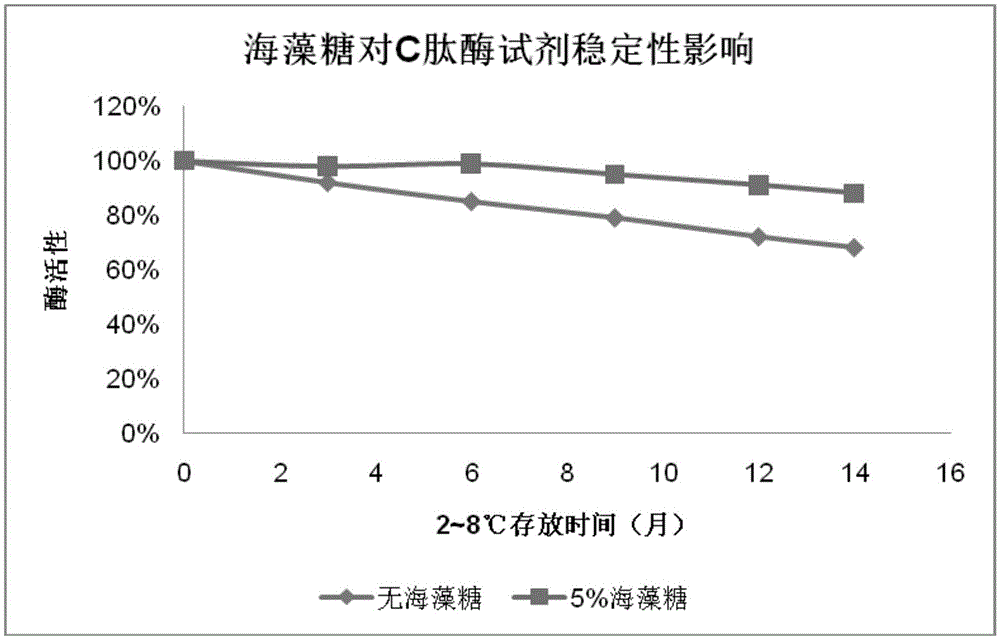
[0062] The C-peptide monoclonal antibody cross-linked magnetic particle of the present invention can be used to detect C-peptide or prepare a C-peptide detection kit, thereby significantly improving the sensitivity of C-peptide detection.
[0063] The term "nonionic surfactant" as used herein has the meaning commonly understood by those of ordinary skill in the art, ie, a surfactant that does not generate ions in aqueous solution. Nonionic surfactants do not dissociate when dissolved in water, and the lipophilic groups in their molecules are r...
Embodiment 1
[0135] Example 1. Preparation of C-peptide monoclonal antibody cross-linked magnetic particles
[0136] 1) Take 10 mg of 1.0 μm magnetic particles whose active functional groups are carboxyl groups, and wash them twice with 50 mM MES buffer at pH 6.0;
[0137] 2) Remove the supernatant after magnetic suction, add 0.5mL of 50mM pH6.0 MES buffer and mix well, then add 0.5mL of 25mg / mL carbodiimide (EDC) solution and mix well;
[0138] 3) react at room temperature for 30 minutes;
[0139] 4) Remove the supernatant after magnetic suction, and wash twice with 50mM pH6.0 MES buffer;
[0140] 5) Add 0.05 mg of the C-peptide monoclonal antibody obtained in Example 1, then dilute to 1 mL with 50 mM MES buffer at pH 6.0, and mix well;
[0141] 6) React overnight at 37°C;
[0142] 7) Wash twice with a solution containing 50 mM pH7.8 Tris-HCl, 150 mM NaCl, and 1% BSA.
[0143] 8) Add 2 mL of a solution containing 50 mM pH7.8 Tris-HCl, 150 mM NaCl, and 1% BSA, and mix well.
[0144] 9...
Embodiment 2
[0148] Example 2. Pretreatment of C-peptide magnetic particle cross-linked product
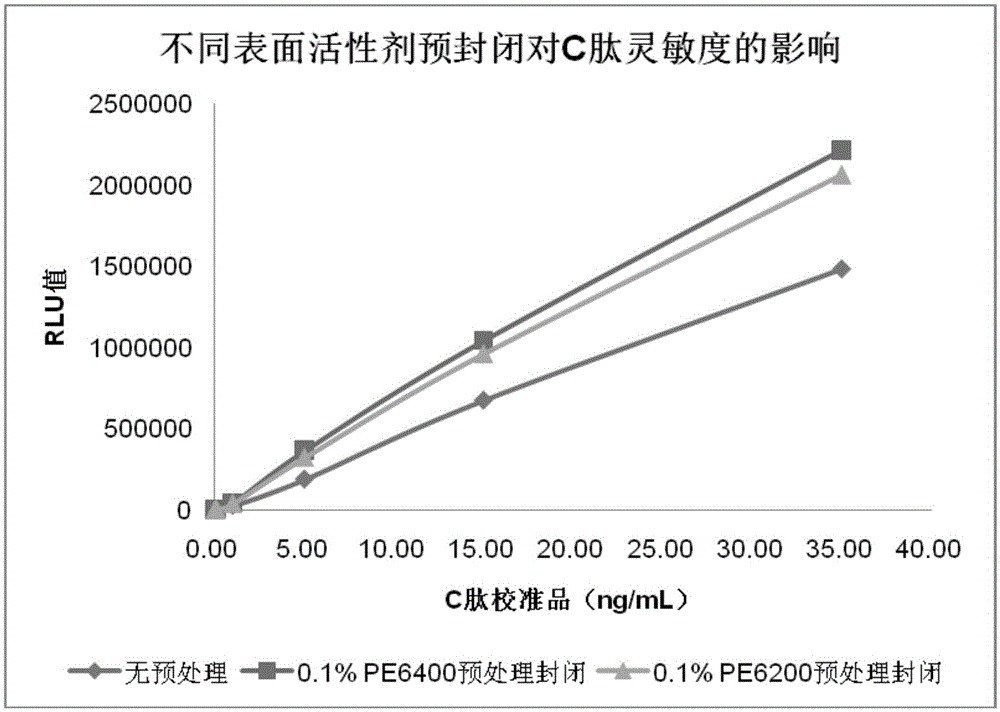
[0149] 1) the nonionic surfactant PE6400 and PE6200, diluted with purified water to 0.1% (mass percentage concentration)
[0150] 2) After the procedure in Example 1.6), magnetically absorb and remove the supernatant.
[0151] 3) Add C-peptide magnetic particle cross-linked product to 0.1% PE6400 solution or 0.1% In the PE6200 solution, the final concentration of the cross-linked magnetic particles is 10mg / mL; or add the cross-linked C-peptide magnetic particles to 0.1% PE6400 solution and 0.1% In the mixed solution of PE6200 solution (volume ratio: 1:1), the final concentration of the cross-linked magnetic particles is 10mg / mL, and mix at 15-30°C for 90 minutes;
[0152] 4) Continue the procedure of Example 1.7) and subsequent steps.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com