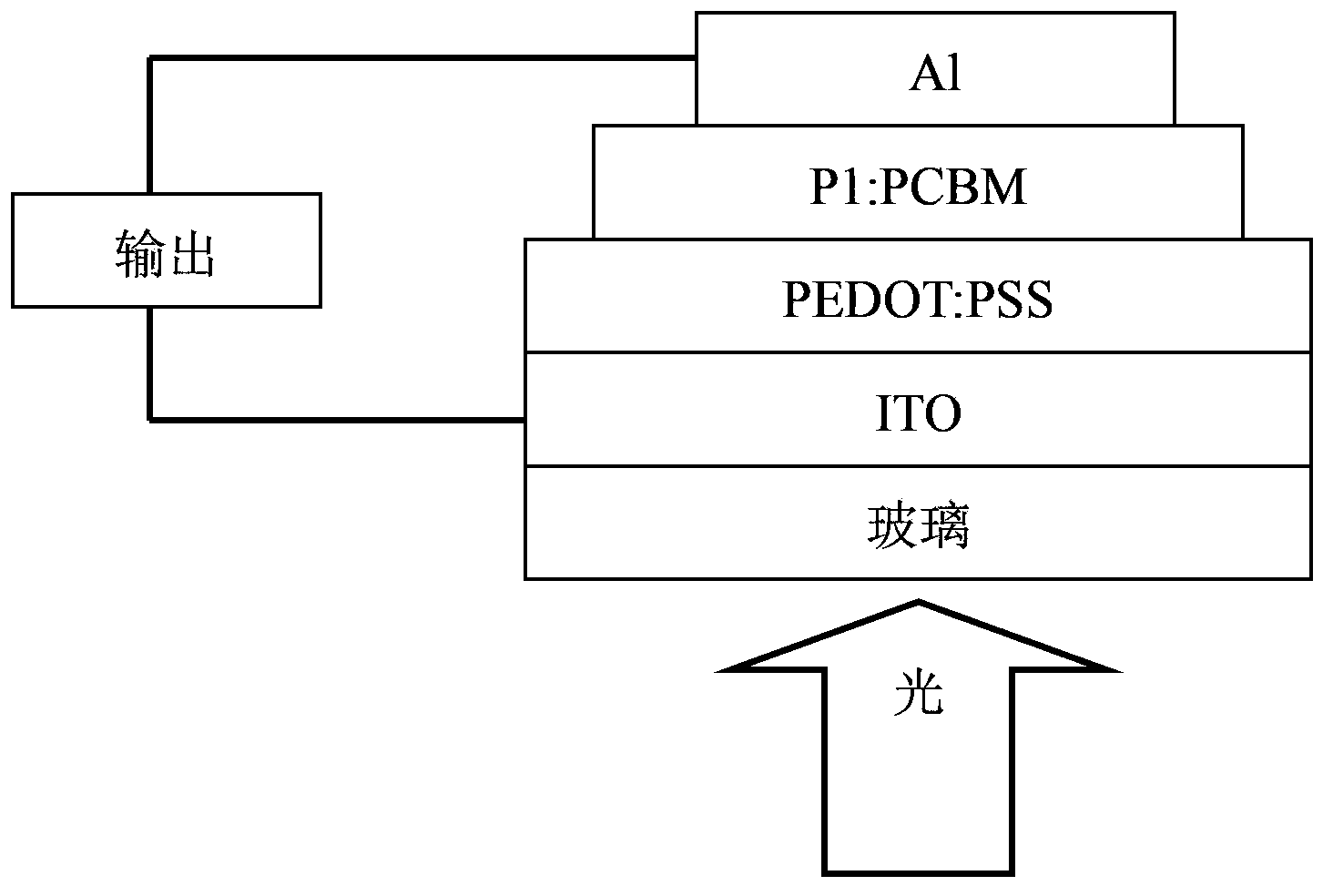
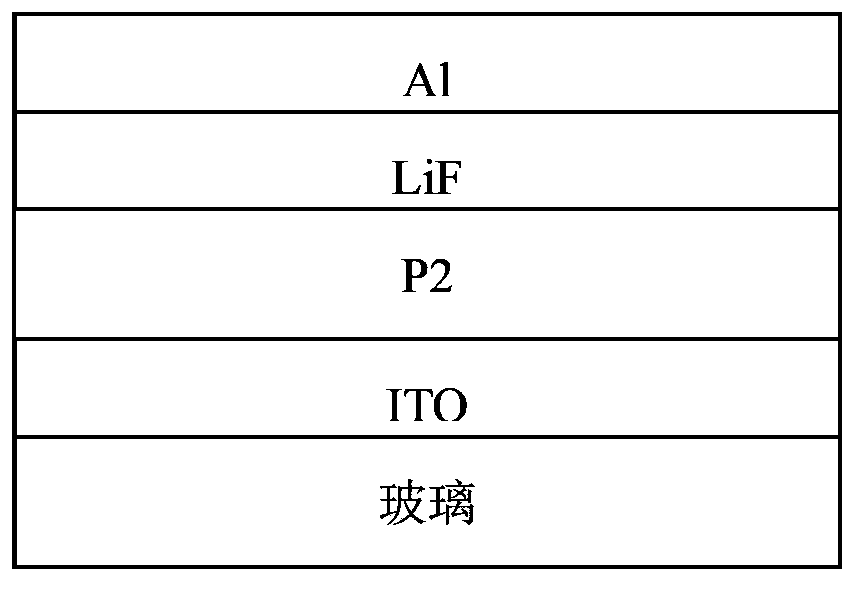
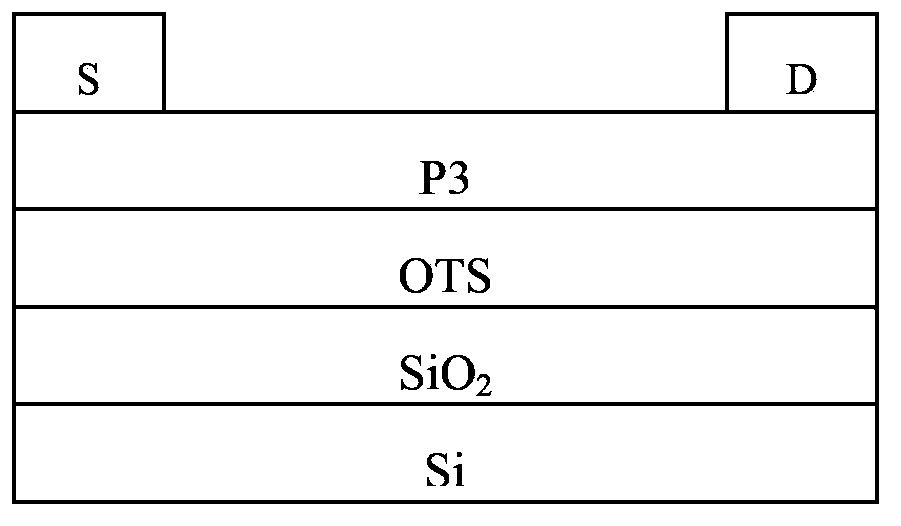
Conjugated polymer, preparation method and applications thereof
A technology of conjugated polymers and compounds, applied in chemical instruments and methods, semiconductor/solid-state device manufacturing, organic chemistry, etc., to achieve the effect of increasing the light absorption range and reducing the energy gap
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The present invention also includes the preparation method to above-mentioned conjugated polymer, and this method comprises the following steps:
[0038] a) provide the following compounds A and B;
[0039] Compound A is
[0040] Compound B is
[0041] b) Under an inert gas atmosphere, add the compound A and the compound B into the organic solvent at a molar ratio of 1:1.1 to 1.5, remove the residual oxygen by bubbling, then quickly add the catalyst, and remove the residual oxygen by bubbling again , carry out the Stille coupling reaction at 60-120°C for 24-72 hours, separate and purify to obtain the conjugated polymer (P);
[0042]
[0043] Among them, R 1 for C 6 ~C 15 the alkyl group, R 2 for H or C 1 ~C 16 Alkyl, R 3 for C 1 ~C 17 Alkyl group, n is a natural number between 5 and 60. The reaction formula is as follows:
[0044]
[0045] The catalyst may be tetrakis(triphenylphosphine)palladium or bis(triphenylphosphine)palladium dichloride; or ...
Embodiment 1
[0059] The conjugated polymer disclosed in this example is specifically compound P1, and its structural formula is as follows:
[0060]
[0061] One, the preparation of compound A1:
[0062] 1) Synthesis of 2,3-dibromo-5-nonanoylthiophene (b1)
[0063] In an ice-salt bath, 2.42g (10mmol) 2,3-dibromothiophene (a), 1.95g (11mmol) nonanoyl chloride and 20ml dichloromethane were successively added to the reaction flask, cooled to 0°C, and 1.73 After dissolving g (13 mmol) of aluminum trichloride with 10 ml of dichloromethane, it was slowly dropped into the reaction mixture through a constant pressure dropping funnel. After the dropwise addition was completed, stir at 0° C. for 2 h, then slowly pour 40 ml (2 mol / L) of glacial hydrochloric acid into the solution to quench the reaction. After the reaction solution was extracted with ether, dried over anhydrous magnesium sulfate, and the organic solvent was removed by rotary evaporation, the crude product was obtained. After vacu...
Embodiment 2
[0078] The conjugated polymer disclosed in this example is specifically compound P2, and its structural formula is as follows:
[0079]
[0080] One, the preparation of compound A2:
[0081] 1) Synthesis of 2,3-dibromo-5-nonanoylthiophene (b2)
[0082] In an ice-salt bath, 2.42g (10mmol) 2,3-dibromothiophene (a), 3.17g (11 mmol) heptadecanoyl chloride and 60ml 1,2-dichloroethane were successively added to the reaction flask to make Cool to 0 °C. After dissolving 1.73g (13mmol) of aluminum trichloride in 10ml of dichloromethane, it was slowly dropped into the reaction mixture through a constant pressure dropping funnel. After the dropwise addition was completed, after stirring at 0° C. for 2 h, slowly pour 40 ml of 2M ice hydrochloric acid to quench the reaction. After the reaction solution was extracted with ether, dried over anhydrous magnesium sulfate, and the organic solvent was removed by rotary evaporation, the crude product was obtained. After vacuum drying, no fur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com