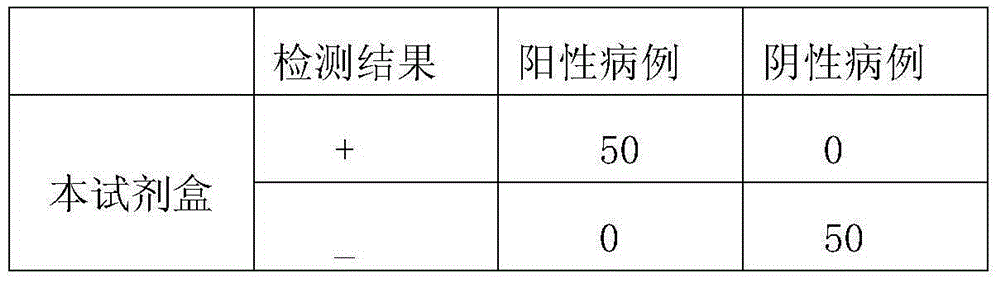
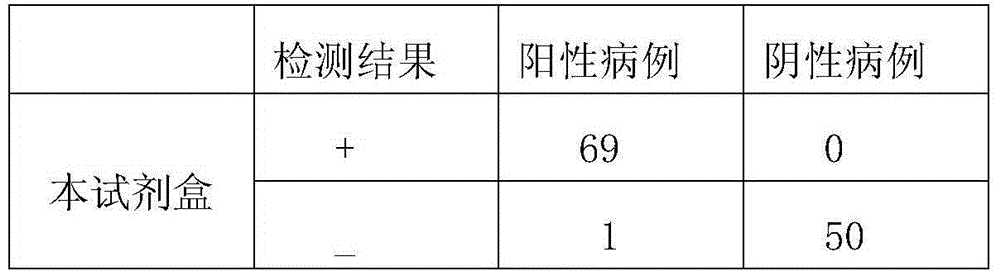
Method and kit for rapid co-examination of anti-human Haemophilus influenzae igm and IgG antibodies based on magnetic separation and multicolor quantum dot labeling
A Haemophilus, magnetic separation technology, applied in the field of medical testing, can solve the problems of long time, increased labor intensity, operational errors, and inability to achieve simultaneous detection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1 Preparation and purification of recombinant human Haemophilus influenzae P6 protein
[0076] 1. Cloning of related genes
[0077] Bioinformatic analysis of human Haemophilus influenzae membrane protein P6 (the accession number in the NCBI protein database is AAA24994) to obtain the peptide with the most abundant antigenic epitope in its extracellular conserved domain, and find its corresponding DNA coding sequence At the same time, the whole gene sequence was chemically synthesized after introducing the restriction site NdeI at the 5' end of the sequence, the termination signal TAA and the restriction site XhoI at the 3' end (the whole sequence synthesis was completed by GenScript Biotechnology Co., Ltd., upon delivery The artificially synthesized gene fragment is connected to the vector pUC57), which is denoted as P6. The full sequence of its gene is shown in the sequence listing. Specifically, the protein sequence encoded by the P6 gene is 48-153aa of the ...
Embodiment 2
[0086] Example 2 Preparation of anti-human Haemophilus influenzae antibody capture nano-magnetic beads
[0087] 1. Optimization of reaction conditions for recombinant P6-His fusion protein coupled to magnetic beads:
[0088] Magnetic beads coupled with recombinant human P6-His fusion protein were used as solid phase carrier, and mouse anti-human IgM monoclonal antibody labeled with quantum dots was used as detection antibody to detect the positive serum of anti-human Haemophilus influenzae IgM antibody. Conjugation of recombinant proteins. A series of optimization options were carried out on the particle size of the magnetic beads, as well as the concentration of EDC / NHS activator, the concentration of conjugated antibody, the coupling time, and the type of blocking agent.
[0089] 1.1 Selection of magnetic bead size
[0090] Select carboxyl nano-magnetic beads with a particle size of 50nm, 180nm, 350nm, 1150nm, and 3μm, add PBS buffer containing 4mg / mlEDC and 4mg / mlNHS for ...
Embodiment 3
[0102] Example 3 Preparation of anti-human IgM and IgG nanoprobes labeled with multicolor quantum dots respectively
[0103] 1. Optimization of the reaction conditions for nanocarboxyl quantum dot-labeled mouse anti-human IgM monoclonal antibody:
[0104] 1.1. Determination of the optimal labeling pH of the carboxyl quantum dot-labeled antibody probe
[0105] The pH of the phosphate buffer in the labeling reaction was set to 5, 6, 7, 8, and 9 respectively, and the fluorescence intensity of the labeled product was measured with a full spectrometer, and the influence of different pH values on the coupling reaction was observed, and the quantum dot-labeled monoclonal antibody was determined. The optimum pH for the reaction is 7.0-8.0. This experiment chooses pH7.4.
[0106] 1.2. Determination of the optimal labeling amount of carboxy quantum dot-labeled antibody probes
[0107] Set the ratio of quantum dot molar concentration to monoclonal antibody concentration to 1:1, 1:2,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com