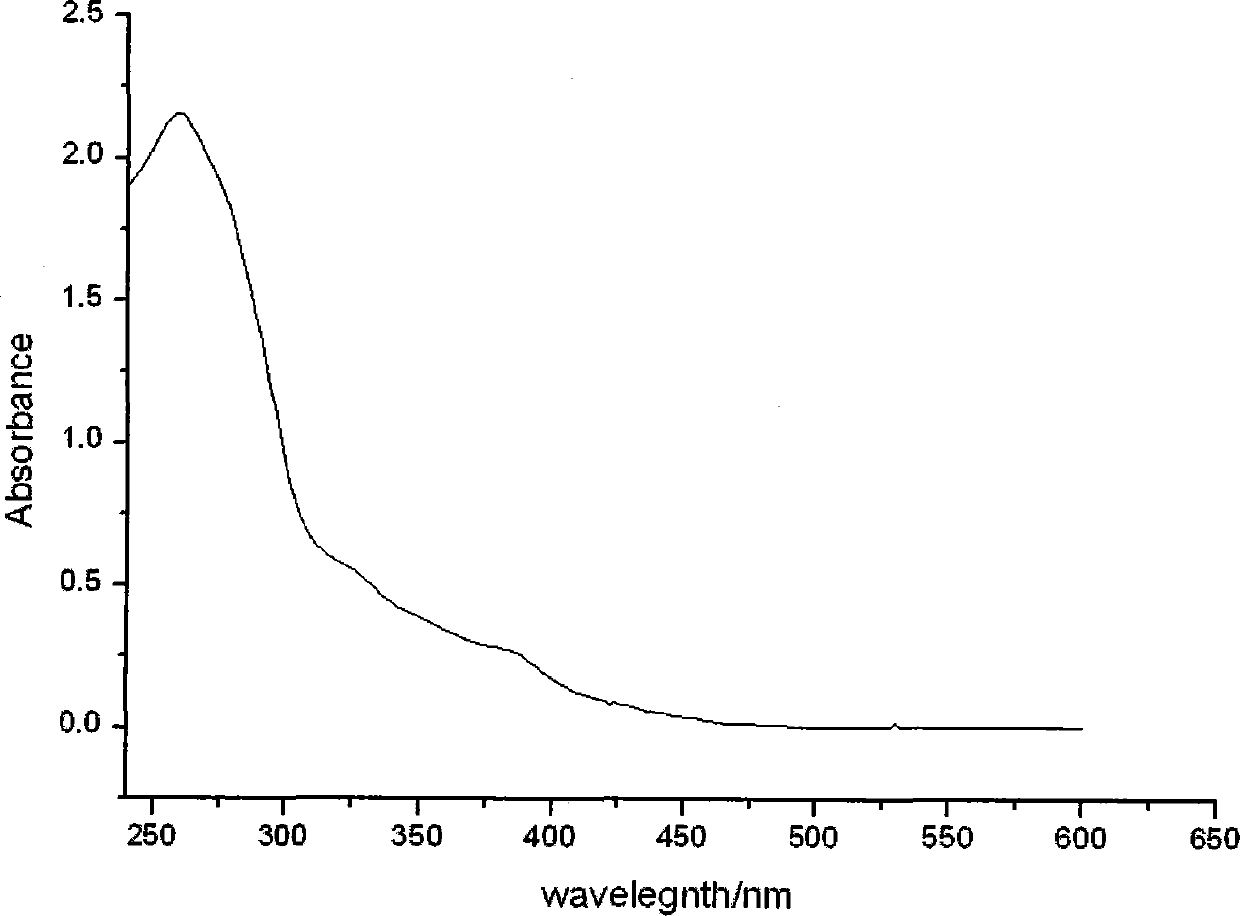
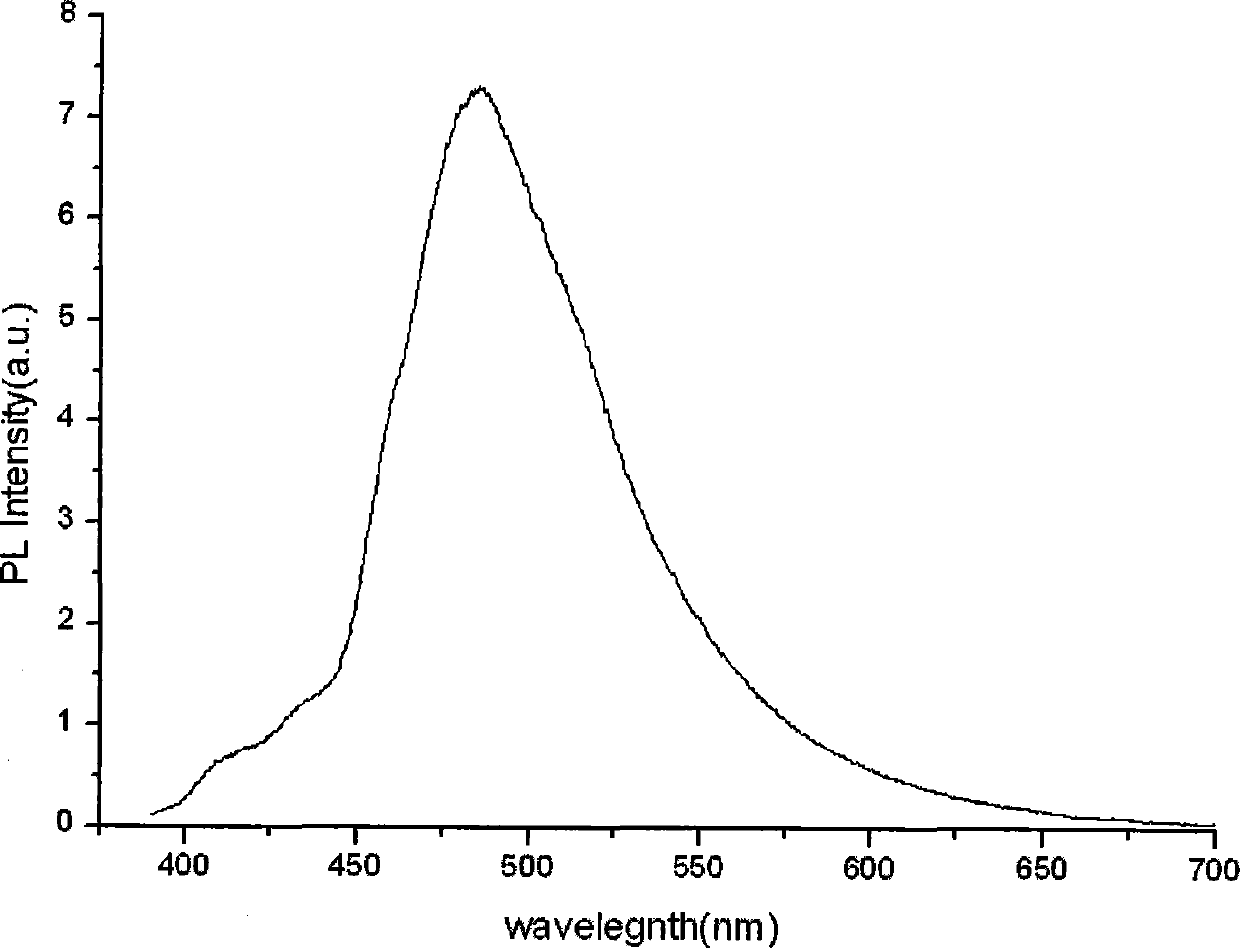
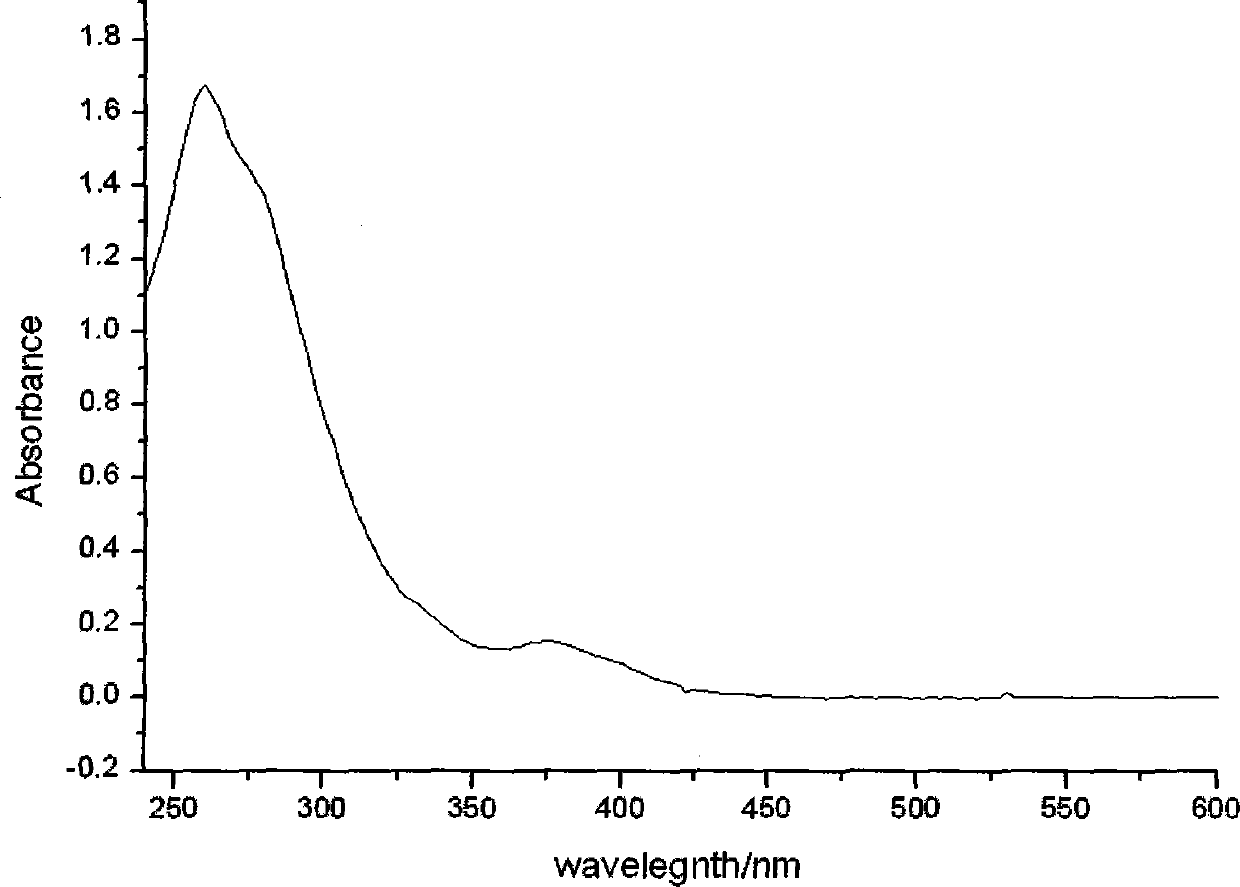
Ionic iridium complex with pyrimidine ligand and preparation method thereof
A technology of iridium complexes and pyrimidines, applied in the field of preparation of pyrimidine iridium complexes, can solve the problems of less reports of ionic complexes, poor performance, etc., to improve energy transfer efficiency, increase interaction, long life effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0042] Synthesis of 2-(2,4-difluorophenyl)pyrimidine (DFPPM)
[0043] Weigh 3.996g (25mmol) 4,6-dimethyl-2-chloropyrimidine, 4.7453g (30mmol) 2,4-difluorophenylboronic acid, 0.6794g (2.5mmol) triphenylphosphine in a 100ml three-necked flask In, add 25ml ethylene glycol dimethyl ether, 35ml potassium carbonate (9.315g) solution. Nitrogen was passed at room temperature for 30 minutes, and then 0.1427 g (0.625 mmol) of palladium acetate was added. Magnetic stirring, reflux at 118°C for 18 hours. The reaction was stopped, cooled to room temperature, the organic phase was separated, the aqueous phase was extracted with ethyl acetate (60ml*4), the combined organic phase was washed with 80ml of water and 80ml of brine, and dried over anhydrous magnesium sulfate. The solvent was removed by rotary evaporation, and the obtained solid was vacuum-dried at 60° C. for 12 hours. The crude product was chromatographed on a silica gel column with dichloromethane / acetone (10:1 volume ratio). ...
example 2
[0045] Synthesis of 4,6-Dimethyl-2-(2,4-difluorophenyl)pyrimidine (MDFPPM)
[0046] Weigh 2.8691g (25mmol) 2-chloropyrimidine, 4.7681g (30mmol) 2,4-difluorophenylboronic acid, 0.6794g (2.5mmol) triphenylphosphine in a 100ml three-necked flask, add 25ml ethylene glycol di Methyl ether, 35ml potassium carbonate (9.315g) solution. Nitrogen was passed at room temperature for 30 minutes, and then 0.1427 g (0.625 mmol) of palladium acetate was added. Magnetic stirring, reflux at 118°C for 18 hours. The reaction was stopped, cooled to room temperature, the organic phase was separated, the aqueous phase was extracted with ethyl acetate (60ml*4), the combined organic phase was washed with 80ml of water and 80ml of brine, and dried over anhydrous magnesium sulfate. The solvent was removed by rotary evaporation, and the obtained solid was vacuum-dried at 60° C. for 12 hours. The crude product was chromatographed on a silica gel column with dichloromethane / acetone (10:1 volume ratio). ...
example 3
[0048] Synthesis of 4,6-Dimethyl-2-phenylpyrimidine (MPPM)
[0049] Weigh 3.996g (25mmol) 4,6-dimethyl-2-chloropyrimidine, 3.5341g (30mmol) phenylboronic acid, 0.6794g (2.5mmol) triphenylphosphine in a 100ml three-necked flask, add 25ml ethylene di Alcohol dimethyl ether, 35ml potassium carbonate (9.315g) solution. Nitrogen was passed at room temperature for 30 minutes, and then 0.1427 g (0.625 mmol) of palladium acetate was added. Magnetic stirring, reflux at 118°C for 18 hours. The reaction was stopped, cooled to room temperature, the organic phase was separated, the aqueous phase was extracted with ethyl acetate (60ml*4), the combined organic phase was washed with 80ml of water and 80ml of brine, and dried over anhydrous magnesium sulfate. The solvent was removed by rotary evaporation, and the obtained solid was vacuum-dried at 60° C. for 12 hours. The crude product was chromatographed on a silica gel column with dichloromethane / acetone (10:1 volume ratio). After drying, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com