ELISA differential diagnosis kit, method and application of prrsv gene marker vaccine strain
A gene marker and differential diagnosis technology, applied in biological testing, material testing products, measuring devices, etc., can solve the problem of inability to distinguish between wild virus and vaccine virus infection, and achieve the effect of simple structure, small molecular weight and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Determination of Example 1 Antigen Optimal Coating Concentration and Optimum Serum Dilution
[0027] 1. Sera and Reagents
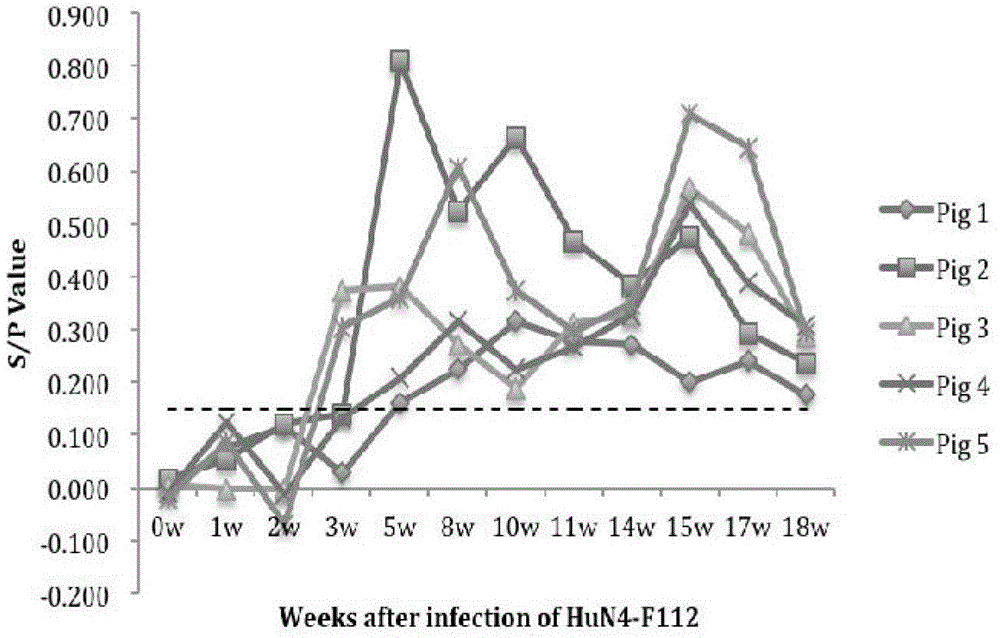
[0028] PRRS negative serum, PRRSV HuN4-F112 positive serum, PRRSVrHN4-Δ25+NP49 strain positive serum, porcine pseudorabies (PRV) positive serum, classical swine fever (CSFV) positive serum and porcine epidemic diarrhea (PEDV) positive serum are all in this experiment Room preservation. Porcine parvovirus (PPV) positive serum and porcine circovirus type 2 (PCV-2) positive serum were provided by the Porcine Disease Laboratory of Harbin Veterinary Research Institute;
[0029] Horseradish peroxidase (HRP)-labeled goat anti-pig IgG was purchased from Sigma Company; skim milk was purchased from BD Company; TMB chromogenic solution, skim milk, and bovine serum albumin (BSA) were purchased from AMRESCO Company. PRRSV antibody detection kit HerdCheckPRRSX3 was purchased from IDEXX company.
[0030] 2. Determination of the optimal coating concentration an...
Embodiment 2
[0036] The determination of embodiment 2 optimal reaction conditions
[0037] 1. Method
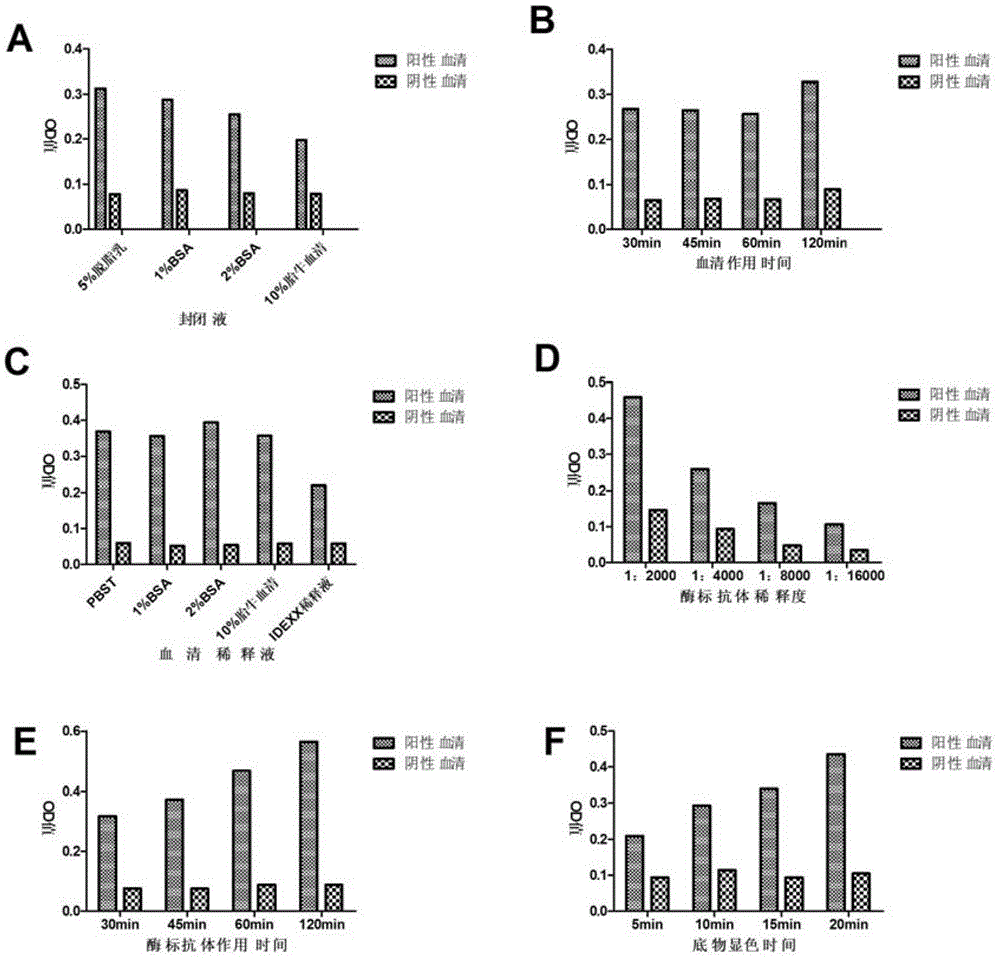
[0038] Optimize the reaction conditions from the aspects of coating time, blocking solution selection, blocking time, serum dilution, enzyme-labeled secondary antibody dilution, substrate reaction time, etc., other conditions remain unchanged, use PRRSHuN4-F112 positive serum and negative serum Carry out indirect 25aa-ELISA assay, read OD with enzyme-linked detector 450 nm value, compare the OD of negative and positive serum in each group 450 nm value and P / N value to determine the best reaction conditions.
[0039] 2. Results
[0040] 2.1 Determination of blocking solution
[0041] Choose 5% skimmed milk, 1% BSA, 2% BSA, 10% fetal bovine serum as the blocking solution. Compare the OD of negative and positive serum in each group 450 nm value and P / N value (Table 2), when 5% skim milk is used as blocking solution, negative serum OD 450 The nm value is the smallest, and the P / N value...
Embodiment 3
[0069] The determination of embodiment 3 critical value
[0070] Collect serum from clinical animal experiments, and measure OD according to the 25aa-ELISA method with optimized conditions 450 nm value, calculate the S / P value of the serum sample according to the following formula:
[0071] S / P=(OD of serum sample to be tested-OD of negative control) / (OD of positive control-OD of negative control).
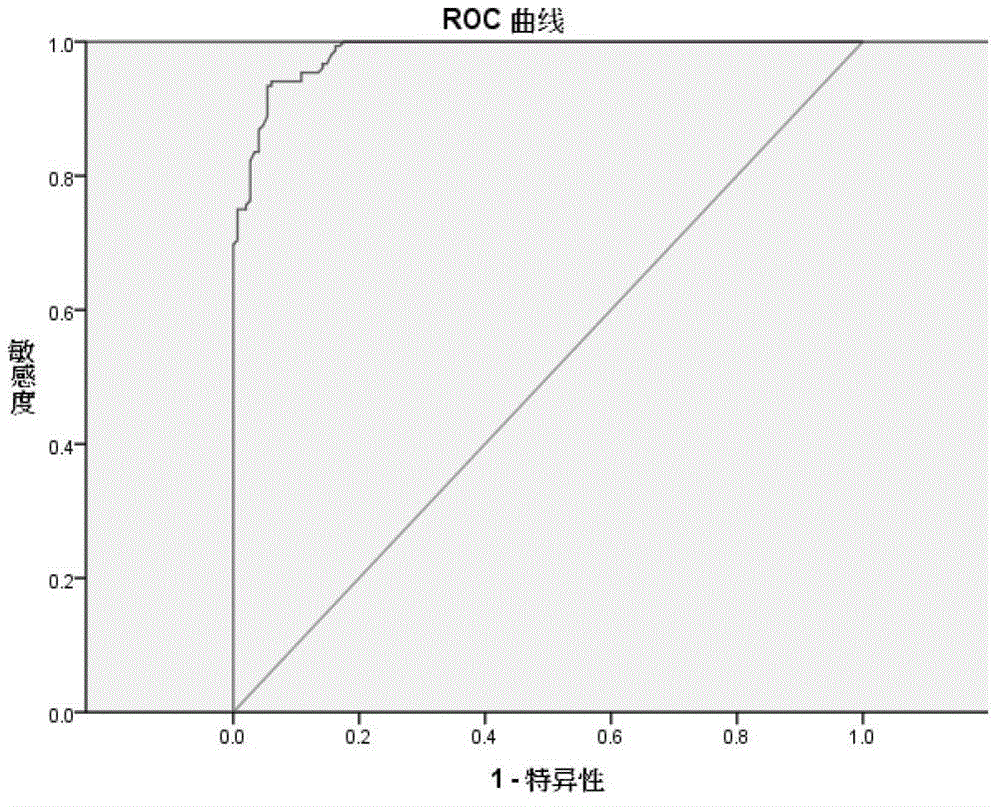
[0072] Samples with S / P value ≥ critical value are judged as positive, and those with S / P value < critical value are judged as negative. The critical value is obtained by ROC statistical analysis method when the sum of sensitivity and specificity values is the largest Corresponding S / P value.
[0073] The ROC curve is a comprehensive index reflecting the continuous variables of sensitivity and specificity. The ROC curve was drawn by SPSS software to obtain the sensitivity and specificity of the 25AA-ELISA detection method. After data processing and analysis, the negative and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dilution degree | aaaaa | aaaaa |
| Dilution degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com