Kit and method for porcine reproductive and respiratory syndrome virus (PRRSV) gene-labeled vaccine strain ELISA differential diagnosis and use of kit
A gene marker and differential diagnosis technology, applied in biological testing, material testing products, measuring devices, etc., can solve the problems of simultaneous existence of vaccines, indistinguishability, loss of aquaculture, etc., and achieve good repeatability, strong specificity, and high sensitivity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 standard serum and the acquisition of clinical serum
[0036] 1. Preparation of recombinant NDV NP49 antigen protein
[0037] (1) Construction of recombinant expression NDV NP49 antigen prokaryotic expression vector
[0038] Design specific primers according to the published NDV La Sota strain (GenBank, NO.AF077761) NP protein gene sequence, upstream primer (NP49-UP): 5'-TTAGAATTCGGGGATGGGGAGACCCAAT-3' (SEQ ID NO: 2), its 5' An EcoR I restriction site is designed at the end; the downstream primer (NP49-DP): 5'-ATACTCG4GATACCCCCAGTCGGTGTCGT-3' (SEQ ID NO: 3), and an Xho I restriction site is designed at the 5' end. The expected amplified length is 165bp. The primers were synthesized by Invitrogen Company (Shanghai). The synthesized primers were diluted with sterilized double distilled water to a final concentration of 10 mmol / L, and stored at -20°C for use.
[0039] Total RNA of NDV La Sota strain was extracted according to the instruct...
Embodiment 2
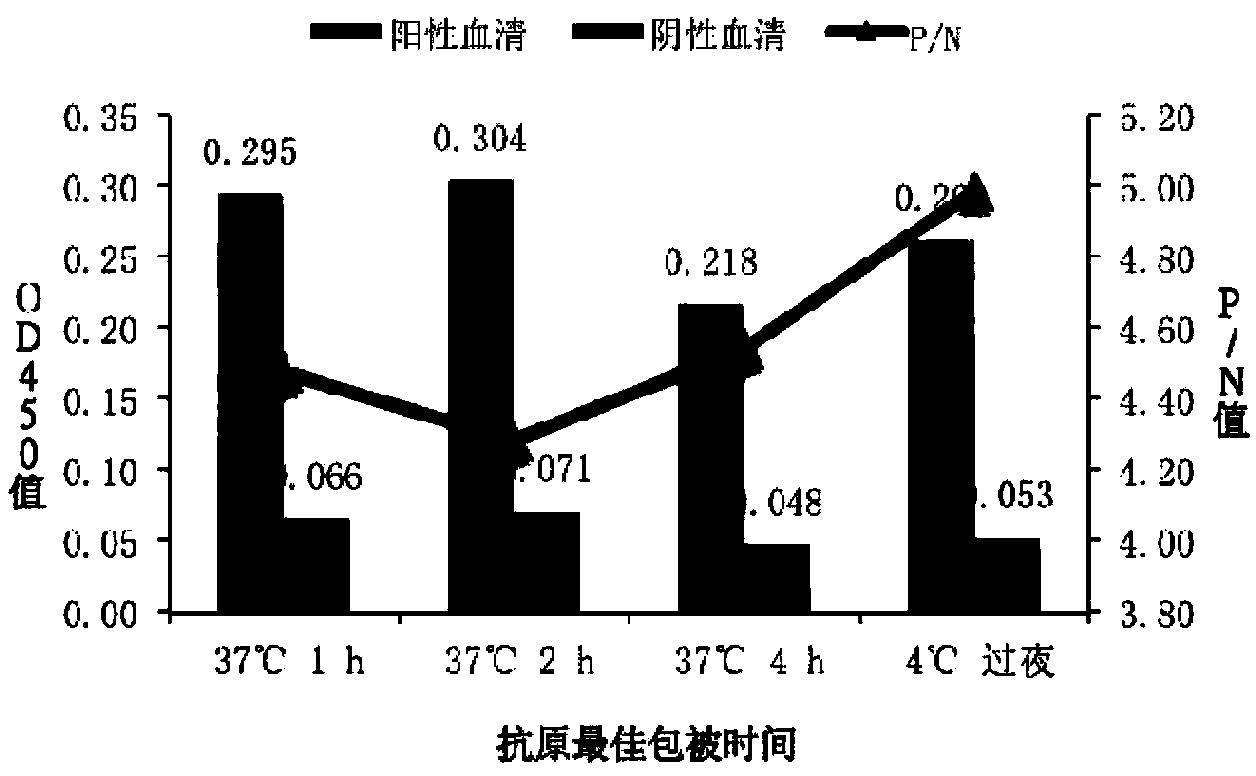
[0055] Embodiment 2 NP49-ELISA experimental condition optimization
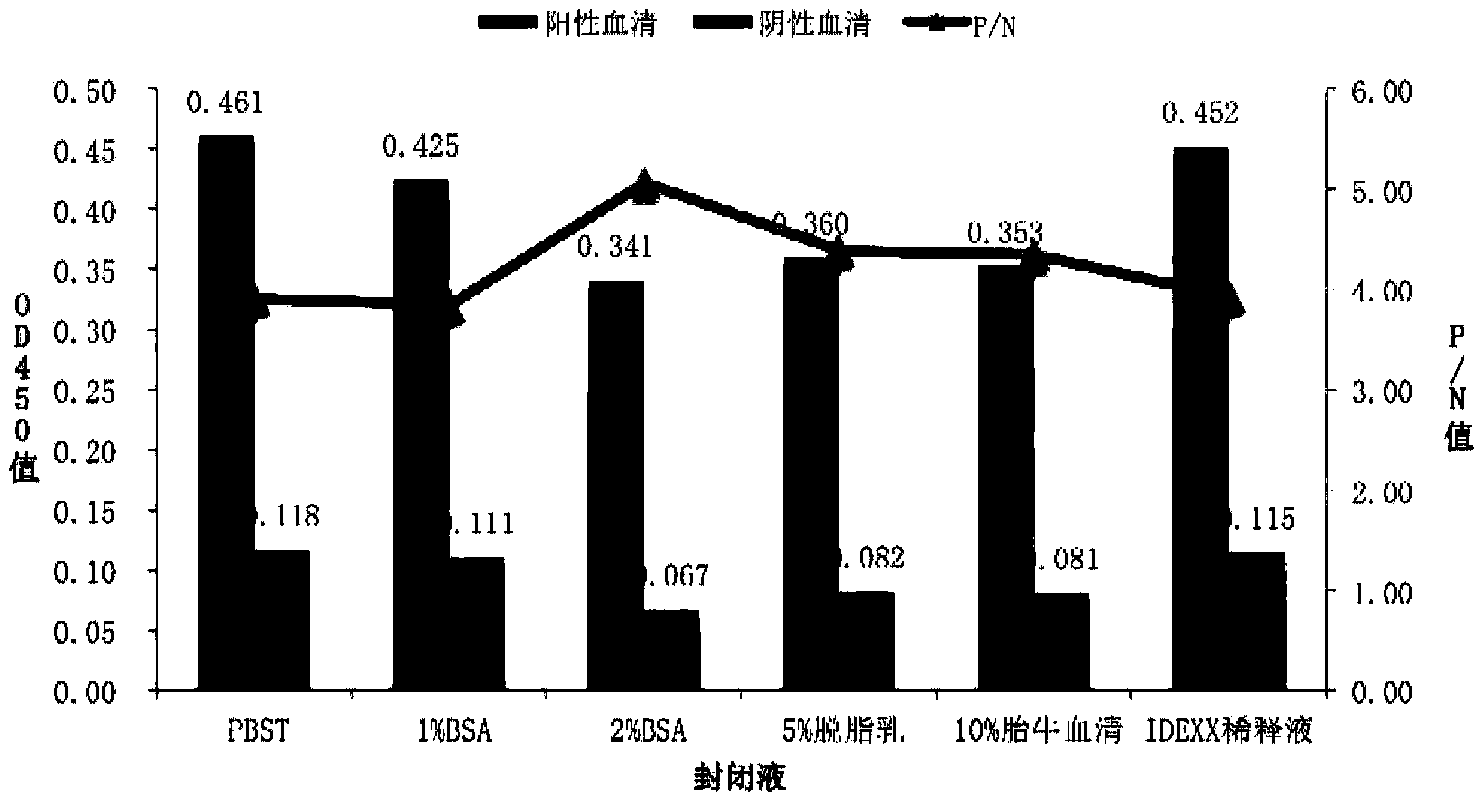
[0056] 1. Determination of the optimal coating concentration and serum dilution of the antigen
[0057] (1) Dilute the 10-fold gradient with 0.05M carbonate buffer (pH9.6), coat the NP49 polypeptide antigen (10 μg / mL to 0.125 μg / mL) on a 96-well plate, 100 μL per well;
[0058] (2) The coating condition is overnight at 4°C, and the sealing is carried out with 5% skimmed milk at 37°C for 2 hours.
[0059] (3) The positive serum and negative serum of PRRSV rHN4-Δ25+NP49 strain were serially diluted at 1:20, 1:40, 1:80, and 1:160, respectively, with 100 μL of dilution per well, and incubated at 37°C for 1 hour for ELISA method. array test.
[0060] (4) Horseradish peroxidase (HRP)-labeled goat anti-pig IgG antibody was diluted 1:10000 times, 100 μL per well, and incubated at 37°C for 1 hour.
[0061] (5) Add 50 μL of TMD chromogenic substrate, and develop color for 10 min at room temperature in the dark.
[...
Embodiment 3
[0092] Embodiment 3 specificity experiment
[0093] The established indirect NP49-ELISA method was used to detect clinically positive sera of CSFV, PRV, PPV, PCV-2, PEDV, and PRRSHuN4-F112. At the same time, PRRSV rHN4-Δ25+NP49 strain positive sera and negative sera were set up as controls to determine the NP49 polypeptide Whether the antigen cross-reacts with sera positive for other common swine diseases.
[0094] Results: OD was determined by detecting CSFV, PRV, PPV, PCV-2, PEDV, PRRS HuN4-F112 positive serum 450 The nm values were 0.049, 0.075, 0.099, 0.044, 0.061, and 0.068, confirming that the NP49 polypeptide antigen had no cross-reaction with other common porcine disease-positive sera.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com