Novel culture method for in vitro differentiation from human embryonic stem cells to functional myocardial cells
A technology of human embryonic stem cells and cardiomyocytes, applied in the field of stem cell biology, can solve the problems of low differentiation rate, unsatisfactory quantity and quality of cardiomyocytes, expensive cytokines, etc., achieve short beating time, promote differentiation efficiency, and prolong beating time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0031] Specific embodiment 1: the formation of embryoid body
[0032] 1. Prepare a gelatin-coated culture dish, the steps include: prepare a 0.1% gelatin solution, spread the gelatin solution on the bottom of a sterile tissue culture dish and incubate for at least 1 hour, then suck off the excess gelatin solution, and then dry it for later use. That is, the gelatin-coated culture dish is prepared.
[0033] 2. Prepare serum-free embryoid body formation medium: 80% DMEM medium for basal medium; add 20% serum substitute, 1mmol / L essential amino acid, 2mmol / LL-glutamine, 0.1mmol / L β-mercaptoethanol , 50IU / mL penicillin, 10μg / mL streptomycin.
[0034] 3. Formation of embryoid bodies: at a concentration of 2×10 5 Individuals / ml inoculated human embryonic stem cells into gelatin-coated culture dishes, adding culture medium and pipetting every day to avoid cell adhesion. CO 2 and 95% saturated humidity. On the 3rd day, embryoid bodies can be formed and suspended in the culture me...
specific Embodiment 2
[0035] Specific embodiment 2: cardiomyocyte differentiation culture
[0036] 1. Preparation of cardiomyocyte differentiation medium: 80% DMEM medium for basal medium, 20% serum substitute, and added inducing substances, respectively: L-glutamine 2mmol / L, L-sodium pyruvate 0.182mmol / L L. Non-essential amino acid 1mmol / L, β-mercaptoethanol 0.1mmol / L, transferrin 5.6mg / L, sodium selenite 20mg / L and p38-MAPK inhibitor 5μmol / L.
[0037] 2. Transfer the cultured embryoid bodies to a 24-well culture plate, place 1 embryoid body in each well, the medium used is the cardiomyocyte differentiation medium, and add nutrient-enhancing substances during the cardiomyocyte differentiation culture process, and at the same time It includes vitamin C, protein hydrolyzate and bovine serum albumin, and its concentrations are vitamin C 0.1mmol / L, protein hydrolyzate 50μg / ml and bovine serum albumin 50μg / ml. Culture conditions are 37°C, 5% CO 2 and 95% saturated humidity. The differentiation mediu...
specific Embodiment 3
[0038] Specific embodiment 3: the differentiation rate experiment of human embryonic stem cells to cardiomyocytes
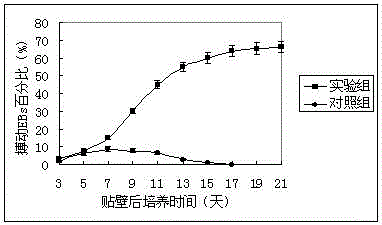
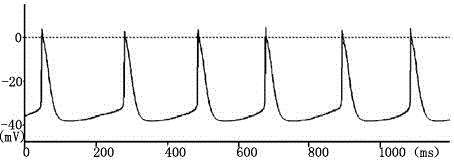
[0039] 1. Experimental steps and methods for differentiating embryoid bodies formed under different conditions into cardiomyocytes: transfer the cultured embryoid bodies to a 96-well culture plate, inoculate one embryoid body in each well, and do 3 experiments in both the experimental group and the control group. plate parallel experiments. Then place at 37°C, 95% saturated humidity and 5% CO 2 Static culture under the conditions; the medium was changed once a day. Among them, the experimental group adopts the embryoid body formed under the condition of suspension culture, and adds nutrition-enhancing substances, and the control group adopts the embryoid body formed under the traditional inverted suspension culture condition, without adding nutrition-enhancing substances; There are spontaneously beating cell clumps, and the presence of rhythmically beating cell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com