Pramipexole dihydrochloride sustained release tablet and preparation method thereof
A technology of pramipexole hydrochloride and sustained-release tablets, which is applied in the field of pharmaceutical preparations, can solve problems such as burst release effects, and achieve the effects of easy administration, small side effects, and great clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
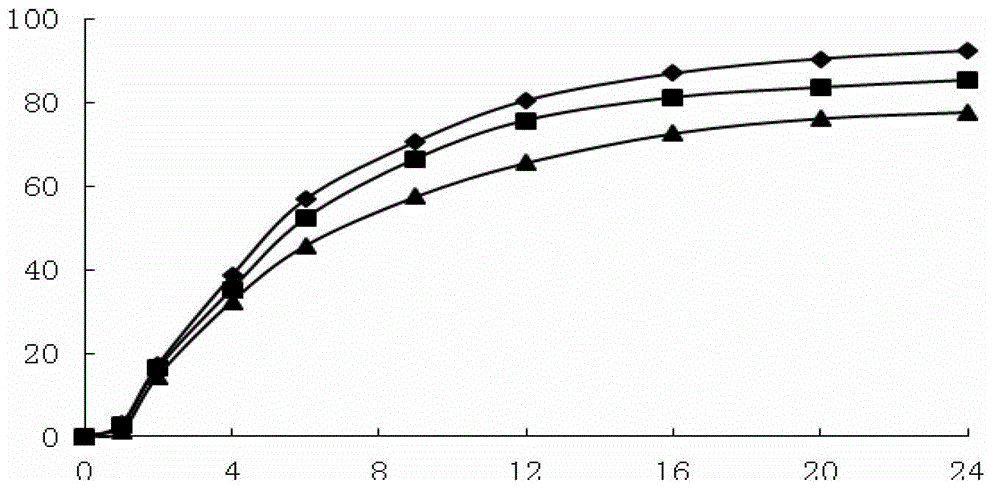
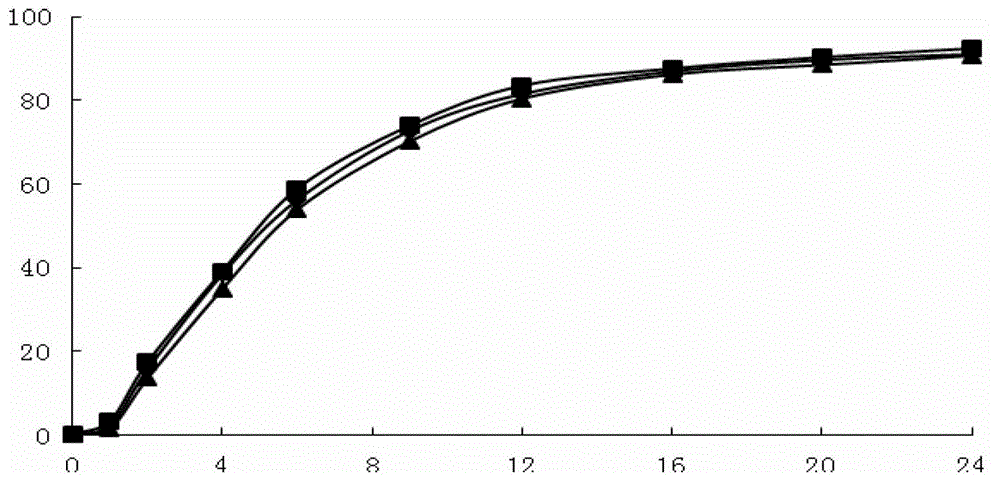
Image
Examples
Embodiment 1
[0042] Embodiment 1: Pramipexole Hydrochloride Sustained Release Tablets
[0043]
[0044] *Removed during coating process
[0045] preparation:
[0046] (1) Mix 22.5g of pramipexole hydrochloride and 22.5g of the same amount of lactose to obtain mixture a, then add 45g of lactose equal to the amount of mixture a and mix to obtain mixture b, until the amount of the mixture is close to the amount of remaining lactose, add The remaining 193.8 g of lactose and 30 g of copolymer of butyl methacrylate, dimethylaminoethyl methacrylate and methyl methacrylate (1:2:1) were mixed.
[0047] (2) Dissolve 12 g of hydroxypropyl cellulose in 228 g of water to form a binder solution, and add the binder solution to the mixture in step (1) for granulation.
[0048] (3) Dry at 50°C in a blast drying oven DHG-9620A.
[0049] (4) Add 1.2 g of silicon dioxide to the dry granules obtained in step (3) and mix.
[0050] (5) Add 3 g of magnesium stearate to step (4) and mix to lubricate the mix...
Embodiment 2
[0055] Embodiment 2: Pramipexole Hydrochloride Sustained Release Tablets
[0056]
[0057] *Removed during coating process
[0058] (1) Mix 1.875g of pramipexole hydrochloride and 1.875g of the same amount of lactose to obtain mixture a, then add 3.75g of lactose equivalent to mixture a and mix to obtain mixture b, until the amount of the mixture is close to the amount of remaining lactose, Add remaining lactose and mix.
[0059] (2) Dissolve 12 g of hydroxypropyl cellulose in 228 g of water to form a binder solution, and add the binder solution to the mixture in step (1) for granulation.
[0060] (3) Dry the wet granules at 50°C in a blast drying oven DHG-9620A.
[0061] (4) Add 1.2 g of silicon dioxide to the dry granules obtained in step (3) and mix.
[0062] (5) Add 3 g of magnesium stearate to step (4) and mix to lubricate the mixture in step (4).
[0063] (6) Put the final mixture in step (5) into a tablet press and press to make 5000 tablet cores.
[0064] (7) D...
Embodiment 3
[0067] Embodiment 3: Pramipexole Hydrochloride Sustained Release Tablets
[0068]
[0069]
[0070] *Removed during coating process
[0071] The preparation of the tablet core is basically the same as in Example 2, except that the coating formulation is different and the prepared coated tablet is not perforated.
[0072] The amount of pramipexole hydrochloride in each tablet was measured to be 0.360mg, equivalent to 96.0% of the theoretical content.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com