Anti-Adrenomedullin (ADM) antibody or anti-ADM antibody fragment or an anti-ADM non-Ig protein scaffold for use in therapy
A technology of adrenomedullin and antibody fragments, applied in the direction of antibodies, antibody medical components, anti-hormone immunoglobulin, etc., can solve harmful and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0568] Production of Antibodies and Determination of Their Affinity Constants
[0569] Several human and murine antibodies were produced and their affinity constants determined (see Tables 1 and 2).
[0570] Peptides / conjugates used for immunization:
[0571] Peptides for immunization were synthesized, see Table 1 (JPT Technologies, Berlin, Germany), with an additional N-terminal cysteine (if there is no cysteine in the selected ADM sequence) residue The peptide is conjugated to bovine serum albumin (BSA). Peptides were linked to BSA by using Sulfolink coupling gels (Perbio-science, Bonn, Germany). The coupling protocol was performed according to the Perbio manual.
[0572] Raise mouse antibodies as follows:
[0573] 100 μg peptide-BSA-conjugate (emulsified in 100 μl Freund’s complete adjuvant) on days 0 and 14 and 50 μg peptide-BSA-conjugate (in 100 μl Freund’s adjuvant) on days 21 and 28 in complete adjuvant) to immunize Balb / c mice. Three days before fusion experi...
Embodiment 2
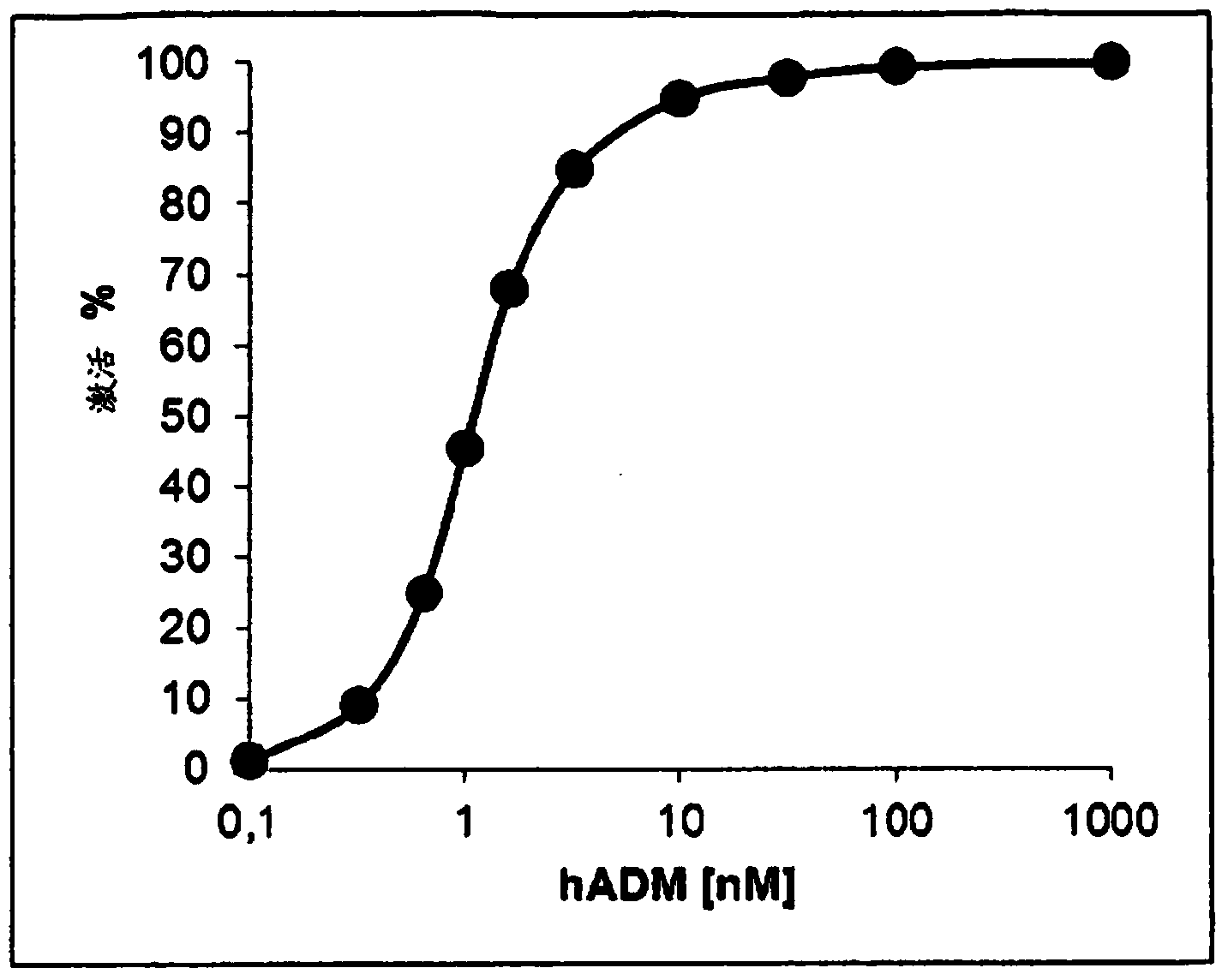
[0630] Effect of selected anti-ADM antibodies on anti-ADM biological activity
[0631] The effect of selected anti-ADM antibodies on ADM biological activity was tested in a human recombinant adrenomedullin receptor cAMP functional assay (adrenomedullin bioassay).
[0632] Testing of Antibodies Targeting Human or Mouse Adrenomedullin in the Human Recombinant Adrenomedullin Receptor cAMP Functional Assay (Adrenomedullin Bioassay)
[0633] Material:
[0634] Cell line: CHO-K1
[0635] Receptor: Adrenomedullin (CRLR+RAMP3)
[0636] Receptor accession number cell line: CRLR: U17473; RAMP3: AJ001016
[0637] Before testing, CHO-K1 cells expressing human recombinant adrenomedullin receptor (FAST-027C) grown in antibiotic-free medium were isolated by gently washing with PBS-EDTA (5 mM EDTA) and recovered by centrifugation , and resuspended in assay buffer (KRH: 5mM KCl, 1.25mM MgSO 4 , 124mM NaCl, 25mM HEPES, 13.3mM Glucose, 1.25mM KH 2 PO 4 ,1.45mM CaCl 2 ,0.5g / l BSA).
[06...
Embodiment 3
[0648] Stabilization of hADM data by anti-ADM antibody
[0649] The hADM immunoassay was used to test the stabilizing effect of human ADM antibodies on human ADM.
[0650] Immunoassay for quantification of human adrenomedullin
[0651] The technique used is a luminescent immunoassay based on acridinium ester-labeled sandwich-coated tubes.
[0652] Labeled compound (tracer): mix 100μg (100ul) CT-H (1mg / ml in PBS, pH7.4, AdrenoMed AGGermany) with 10μl acridinium NHS-ester (Acridinium NHS-ester) (1mg / ml in Acetonitrile, InVent GmbH, Germany) (EP0353971) was mixed and incubated at room temperature for 20 min. exist Labeled CT-H was purified by gel filtration HPLC on SEC400-5 (Bio-Rad Laboratories, Inc., USA). The purified CT-H was diluted in (300mmol / L potassium phosphate, 100mmol / L NaCl, 10mmol / L Na-EDTA, 5g / L bovine serum albumin, pH 7.0). The final concentration is about 800.000 relative light units (RLU) of labeled compound (about 20 ng of labeled antibody) per 200 μL. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com