A lithium-sulfur battery
A lithium-sulfur battery and electrolyte technology, which is applied in the field of new lithium-sulfur battery systems, can solve the problems of reduced electrolyte conductivity, reduced battery performance, and loss of positive active materials, achieving improved cycle stability, improved battery efficiency, and reduced churn effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Prepare a lithium chloride / dimethyl sulfoxide electrolyte solution with a concentration of 1mol / L; place the Celgard2325 microporous membrane at room temperature in a Tris buffer solution of 10mmol / L dopamine / methanol / pH=9.5 (Tris buffer solution of methanol / pH=9.5 Buffer solution (volume ratio: 1:1) was immersed in the solution for 24 hours, washed with methanol, and dried overnight at 60°C under vacuum. Lithium sheet negative electrode is used, the electrolyte prepared above, the treated separator and carbon / sulfur compound are used as the positive electrode (polytetrafluoroethylene as the binder) to assemble a lithium-sulfur button battery, and it is charged and discharged at a rate of 0.05C at about 30°C. The obtained voltage-capacity as figure 1 As shown, the discharge platform is 2.0V, the charging voltage is 2.2V, and the first discharge capacity is 1321mAh / g. It is demonstrated that LiCl / DMSO electrolyte can support the charge-discharge reaction of lithium-sulf...
Embodiment 2
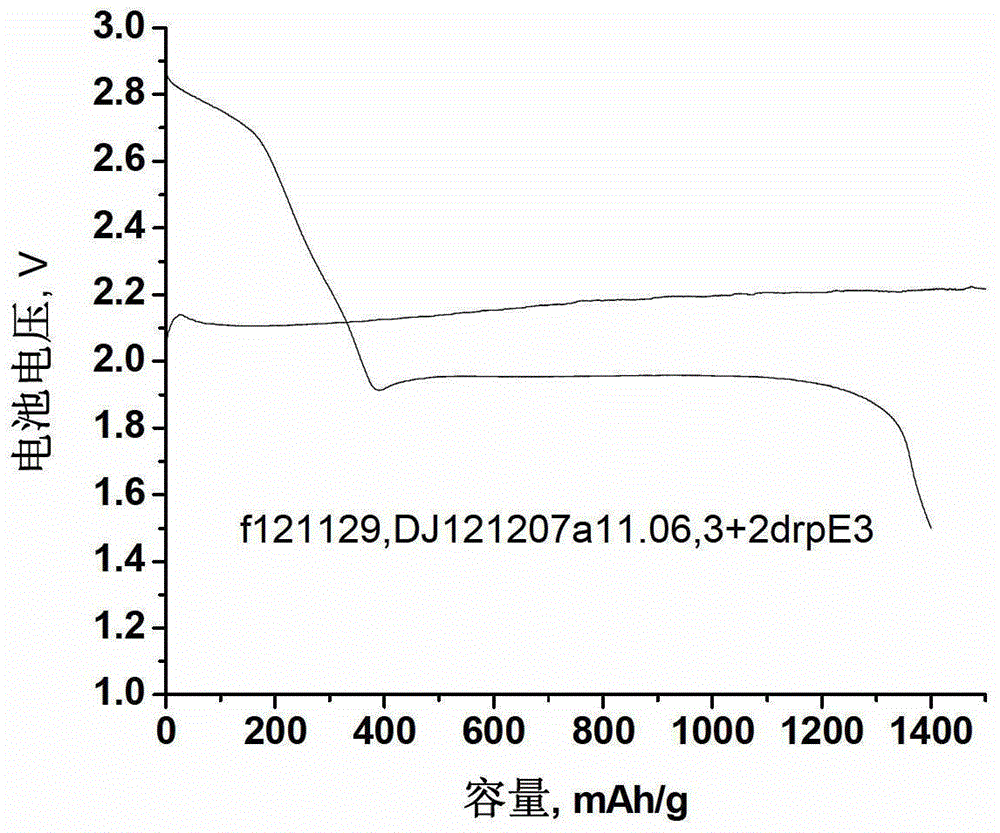
[0032]Celgard2325 was soaked in 10mmol / L dopamine / methanol / pH=9.5 Tris buffer solution (methanol / pH=9.5 Tris buffer volume ratio is 1:1) solution at room temperature for 24 hours, rinsed repeatedly with methanol, vacuumed at 60°C Dry overnight, and then hot press at 85°C and 3MPa for 2 minutes to reduce the pore size. Prepare a lithium chloride / dimethyl sulfoxide electrolyte with a concentration of 1 mol / L. The obtained microporous diaphragm and electrolyte were used to assemble a button battery, the negative pole was a lithium sheet, and the positive pole was a C / S composite electrode (polytetrafluoroethylene was used as a binder). The battery is charged and discharged at a rate of 0.05C at about 30°C, and the following figure 2 As shown in the voltage-capacity curve, the discharge platform is 1.96V, the charging voltage is 2.2V, and the first discharge capacity is as high as 1400mAh / g, which is better than that of Example 1, which proves that the combination of the micropo...
Embodiment 3
[0034] Celgard2325 was soaked in 10mmol / L dopamine / methanol / pH=9.5 Tris buffer solution (methanol / pH=9.5 Tris buffer volume ratio is 1:1) solution at room temperature for 24 hours, rinsed repeatedly with methanol, vacuumed at 60°C Dry overnight, and then hot press at 85°C and 3MPa for 2 minutes to reduce the pore size. Prepare a lithium bromide / dimethyl sulfoxide electrolyte with a concentration of 1 mol / L. The obtained microporous diaphragm and electrolyte were used to assemble a button battery, the negative pole was a lithium sheet, and the positive pole was a C / S composite electrode (polytetrafluoroethylene was used as a binder). The battery is charged and discharged at a rate of 0.05C at 30°C, and the obtained voltage-capacity curve is as follows image 3 As shown, the discharge platform is 1.98V, the charging voltage is 2.2V, the first discharge capacity reaches 1282mAh / g, and multiple charge and discharge cycles can be realized.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com