Telmisartan capsule and preparation method thereof
A kind of technology of telmisartan and capsule
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
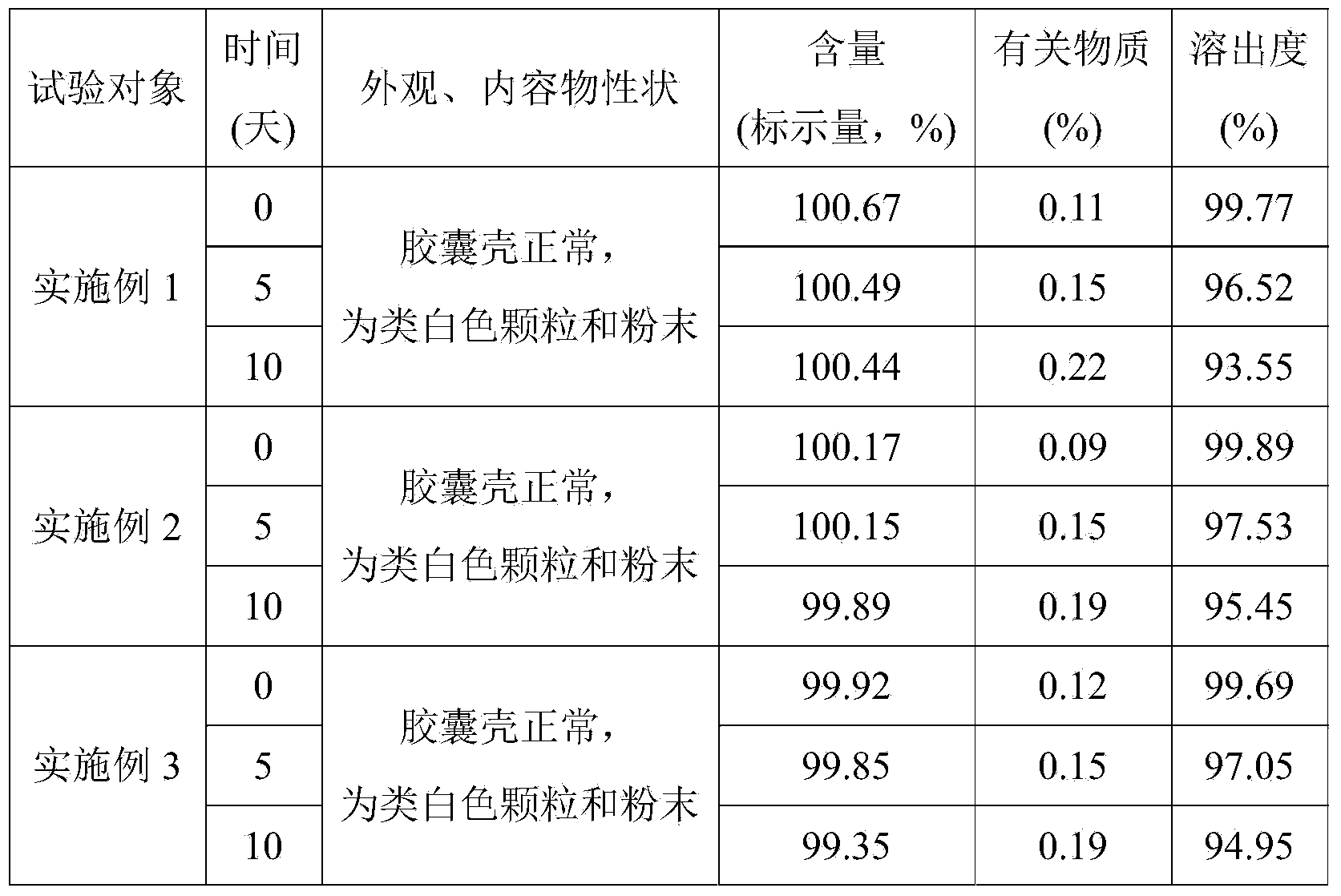
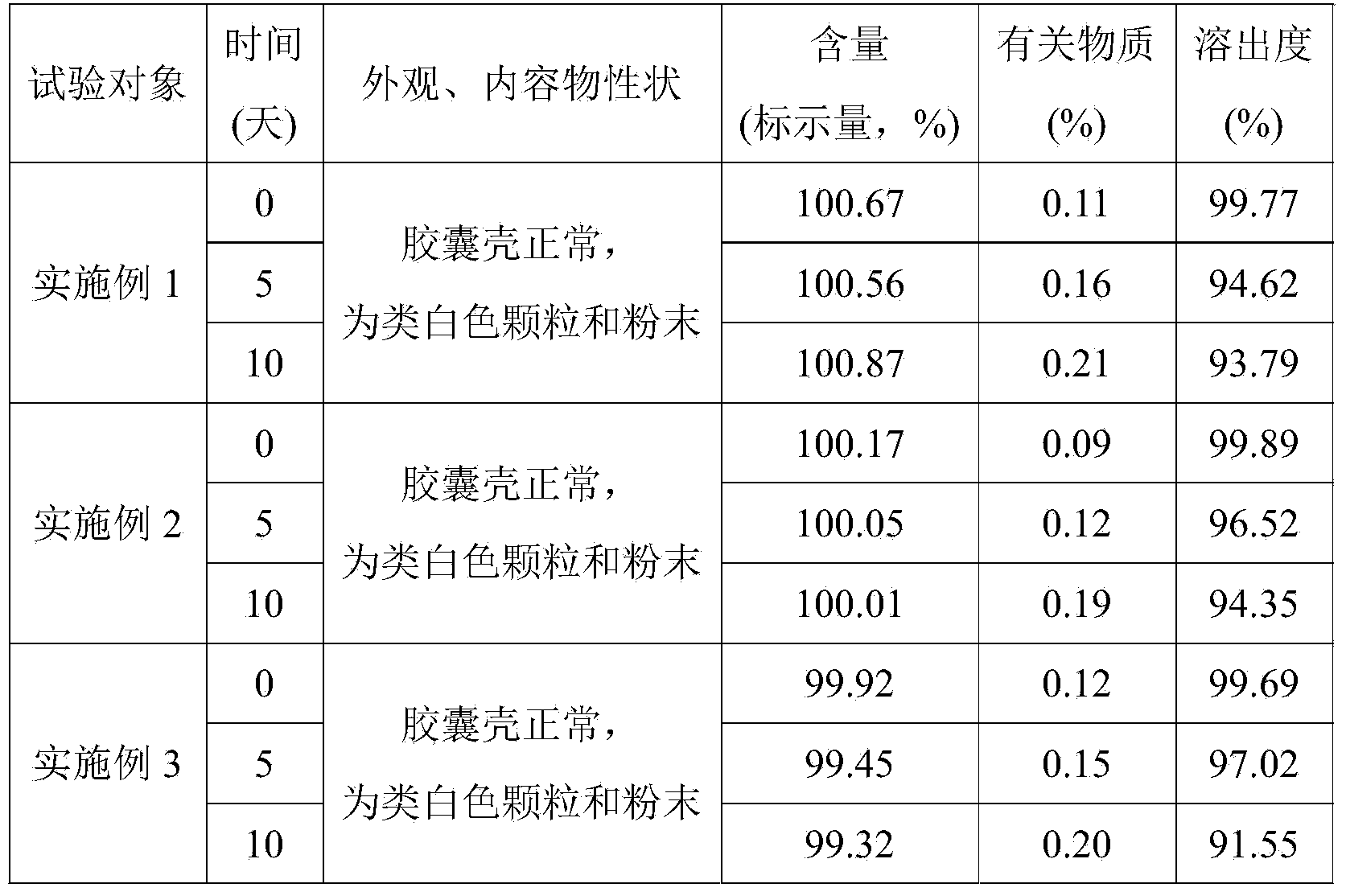
Embodiment 1
[0029] The telmisartan capsule of the present embodiment, every 1000 described telmisartan capsules comprise following components: telmisartan 40g, lactose 210g, sodium hydroxide 3.2g, meglumine 6.0g, polysaccharide Ethanol solution of Vitone K30 25ml.
[0030] The capsules are hard capsules.
[0031] The preparation method of the telmisartan capsule of the present embodiment comprises the following steps:
[0032] 1) Dissolve 3.2 g of sodium hydroxide and 6.0 g of meglumine in a small amount of 50% ethanol aqueous solution, add telmisartan, mix and grind to obtain mixture A;
[0033] 2) Add 210g of lactose to mixture A, mix and dry at 50°C for 60 minutes, then pulverize and pass through a 40-mesh sieve to obtain a mixed fine powder;
[0034] 3) Add 25ml of ethanol solution of povidone K30 with a mass concentration of 5% to the mixed fine powder obtained in step 2) to make a soft material, and pass through a 20-mesh sieve to make wet granules;
[0035] 4) After drying the w...
Embodiment 2
[0038] The telmisartan capsule of the present embodiment, every 1000 described telmisartan capsules comprise following components: telmisartan 40g, lactose 210g, sodium hydroxide 3.2g, meglumine 6.0g, polysaccharide Ethanol solution of Vitone K30 25ml.
[0039] The capsules are hard capsules.
[0040] The preparation method of the telmisartan capsule of the present embodiment comprises the following steps:
[0041] 1) Dissolve 3.2 g of sodium hydroxide and 6.0 g of meglumine in a small amount of 50% ethanol aqueous solution, add telmisartan, mix and grind to obtain mixture A;
[0042] 2) Add 210g of lactose to mixture A, mix and dry at 60°C for 30 minutes, then pulverize and pass through a 40-mesh sieve to obtain a mixed fine powder;
[0043] 3) Add 25ml of ethanol solution of povidone K30 with a mass concentration of 5% to the mixed fine powder obtained in step 2) to make a soft material, and pass through a 20-mesh sieve to make wet granules;
[0044] 4) After drying the w...
Embodiment 3
[0047] The telmisartan capsule of the present embodiment, every 1000 described telmisartan capsules comprise following components: telmisartan 40g, lactose 210g, sodium hydroxide 3.2g, meglumine 6.0g, polysaccharide Ethanol solution of Vitone K30 25ml.
[0048] The capsules are hard capsules.
[0049] The preparation method of the telmisartan capsule of the present embodiment comprises the following steps:
[0050] 1) Dissolve 3.2 g of sodium hydroxide and 6.0 g of meglumine in a small amount of 50% ethanol aqueous solution, add telmisartan, mix and grind to obtain mixture A;
[0051] 2) Add 210g of lactose to mixture A, mix and dry at 55°C for 45 minutes, then pulverize and pass through a 40-mesh sieve to obtain a mixed fine powder;
[0052] 3) Add 25ml of ethanol solution of povidone K30 with a mass concentration of 5% to the mixed fine powder obtained in step 2) to make a soft material, and pass through a 20-mesh sieve to make wet granules;
[0053] 4) After drying the w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com