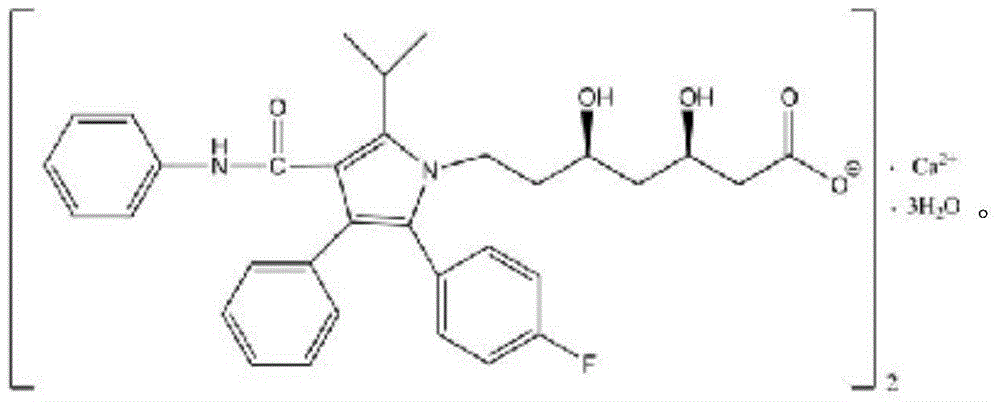
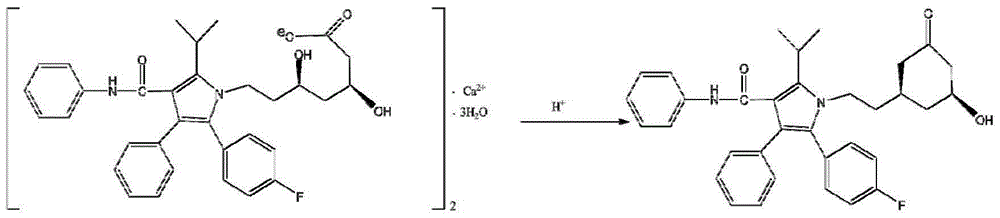
Solid pharmaceutical composition containing atorvastatin calcium
A technology of atorvastatin calcium and its composition, which is applied in the field of medicine, can solve the problems of no lipid-lowering activity and side effects of myalgia, achieve stable quality, reduce the incidence of side effects of myalgia, and achieve the effects of simple prescription and preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Compatibility experiment of auxiliary materials
[0036]Mix atorvastatin calcium raw material, filler microcrystalline cellulose, lactose monohydrate, mannitol, calcium carbonate, and disintegrating agent croscarmellose sodium in a weight ratio of 1:5, and mix the Torvastatin calcium raw material and lubricant magnesium stearate, isolation layer coating powder (hydroxypropyl methylcellulose: polyethylene glycol 2000: sodium lauryl sulfate = 10:1:1 (mass ratio) ) according to the total ratio of 20:1, mix evenly, place them in culture dishes and spread them into thin layers <5mm thick. The sample numbers are A, B, C, D, E, F, G, respectively, and another copy of atorvastatin calcium raw material is placed under the same experimental conditions, numbered H, as a standard reference.
[0037] In addition, since the enteric coating powder is not in direct contact with the tablet core containing atorvastatin calcium raw material, there is no interaction, so it is...
Embodiment 2- Embodiment 6
[0047] Embodiment 2-embodiment 6 (unit: g) prescription design:
[0048] In order to make the tablet core disintegrate quickly after it reaches the small intestine, microcrystalline cellulose is selected as a filler with high porosity and good compressibility. At the same time, to increase the hardness of the tablet core and facilitate coating, lactose monohydrate is selected as the second Two fillers. Also in order to disintegrate quickly after the tablet core reaches the small intestine, croscarmellose sodium with the largest water absorption multiple is selected as the disintegrating agent; in terms of lubricant, a conventional lubricant, magnesium stearate, is selected with a small amount of addition and stable properties. Easy to form into tablets.
[0049] Obtain different doses of atorvastatin calcium enteric-coated tablet prescription and form as follows:
[0050]
[0051] Preparation Process:
[0052] 1) Preparation of tablet core:
[0053] Weigh atorvastatin c...
Embodiment 7
[0058] Example 7 Sample release detection
[0059] Get each 6 of the finished enteric-coated tablets of embodiment 2-embodiment 6, according to Chinese Pharmacopoeia 2010 edition two release assay methods (Appendix X D second method), adopt dissolution assay (Appendix X C first method, Rotating basket method) device, using 750mL of hydrochloric acid solution (9→1000mL) as the release medium, the temperature of the eluate is 37±0.5°C, and the rotation speed is 100 rpm. Operate according to the law. After 120 minutes, immediately lift the rotating basket out of the liquid surface , Discard the hydrochloric acid solution, immediately add 1000ml of phosphate buffer (pH 6.8) preheated to 37°C, continue to operate according to the law, after 45 minutes, take a sample for testing, the results are shown in the following table:
[0060] Table 4 Example 2~Example 6 sample release test results
[0061]
[0062] The above results are in full compliance with the requirements for the re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com