Bovine type A foot-and-mouth disease broad-spectrum multi-epitope vaccine and preparation method and application thereof
A foot-and-mouth disease, multi-epitope technology, applied in chemical instruments and methods, pharmaceutical formulations, recombinant DNA technology, etc., can solve problems such as biosafety acceleration, and achieve the effect of protection against attacks and good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 bovine type A foot-and-mouth disease multi-epitope recombinant antigen
[0030] 1. Bioinformatics analysis of VP1 gene of type A foot-and-mouth disease virus:
[0031] The structural protein VP1 of foot-and-mouth disease virus is the dominant antigen of the virus. Whether it is the isolated and purified natural VP1 protein or the recombinant expression product, it can induce the body to produce protective neutralizing antibodies, which is type-specific. The VP1 gene of type A foot-and-mouth disease virus has a full length of 639 nucleotides, encoding a protein of 213 amino acids, and its main antigenic epitopes are concentrated in the 140-160 amino acid and 200-213 amino acid segments. In this study, the domestic popular strains A / WH / 09, A / XJKT / 58 and GSLX / 62 were compared with the representative strains of bovine type A foot-and-mouth disease virus (NCBI accession number: FJ755067) recently isolated from some countries bordering my count...
Embodiment 2
[0037] Preparation of Example 2 Bovine Type A Foot-and-Mouth Disease Broad-spectrum Multi-epitope Vaccine and Analysis of Immunity Efficacy
[0038] 1. Vaccine preparation:
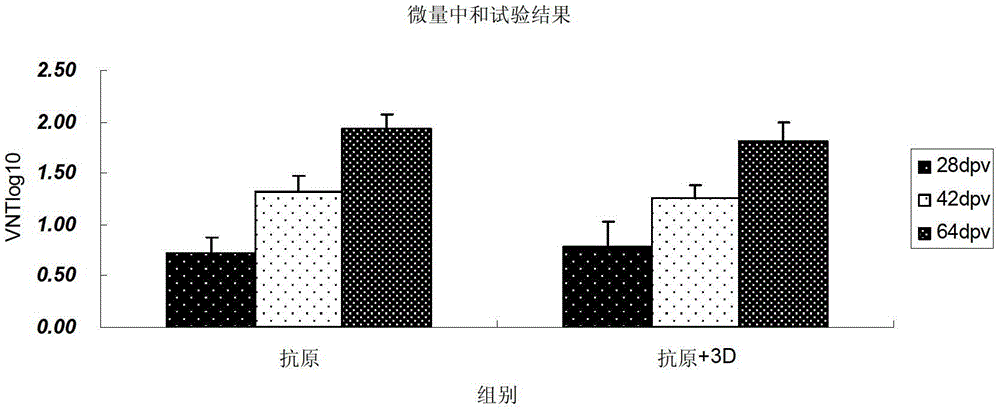
[0039] The purified protein (shown in SEQ ID NO.6, prepared according to the method in Example 1) was quantified by the Bio-Rad quantitative kit and diluted to an appropriate concentration, alone or with the purified 3D protein fragment (amino acid sequence shown in SEQ ID NO.8 The nucleotide sequence encoding it (as shown in SEQ ID NO.7) and the recombinant antigen are configured at a ratio of 1:2 (V / V), and an equal volume of oil adjuvant ISA206 (France) is added to emulsify into a vaccine preparation.
[0040] 2. Guinea pig immune efficacy test:
[0041] Using the prepared vaccine, 0.2ml of each guinea pig was inoculated by intramuscular route (20μg antigen or 20μg antigen + 10μg 3D protein) to immunize 5 guinea pigs respectively. The results of the immunization experiment showed that no matter whethe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com