Chimeric therapeutic anti-CD37 antibodie HH1
A chimeric antibody, CD37 technology, applied in the direction of antibody, radioactive carrier, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0250] The preparation of embodiment 1 chimeric antibody
[0251] Using an expression vector for the V-region gene, a chimeric form of the HH1 antibody was prepared.
[0252] This vector accepts VH and VL chain genes obtained by RT-PCR.
[0253] Then the V-genes were sequenced, and new specific primers were designed to amplify the V-genes, and then they were cloned into the pLNOH2 vector containing human IgG antibody constant region genes (Cγ3, Cγ1 and CH1γ3).
[0254] The pLNOH2 vector comprising the constant regions IgG1CH1 (SEQ ID NO.5), IgG1CH2 (SEQ ID NO.6), IgG1CH3 (SEQ ID NO.7) is shown in SEQ ID NO.8.
[0255] The heavy and light chains of the vector pLNOH2 (SEQ ID NO. 5) and pLNOκ were combined to prepare the combined vector pLNOH2γ1 / καCD37. The entire light chain gene (SEQ ID NO: 9), the CMV promoter and the polyA signal of pLNOκαCD37 were subcloned into pLNOH2hIgG1αCD37.
[0256] In combinatorial vectors, the heavy and light chains are expressed from their own CM...
Embodiment 2
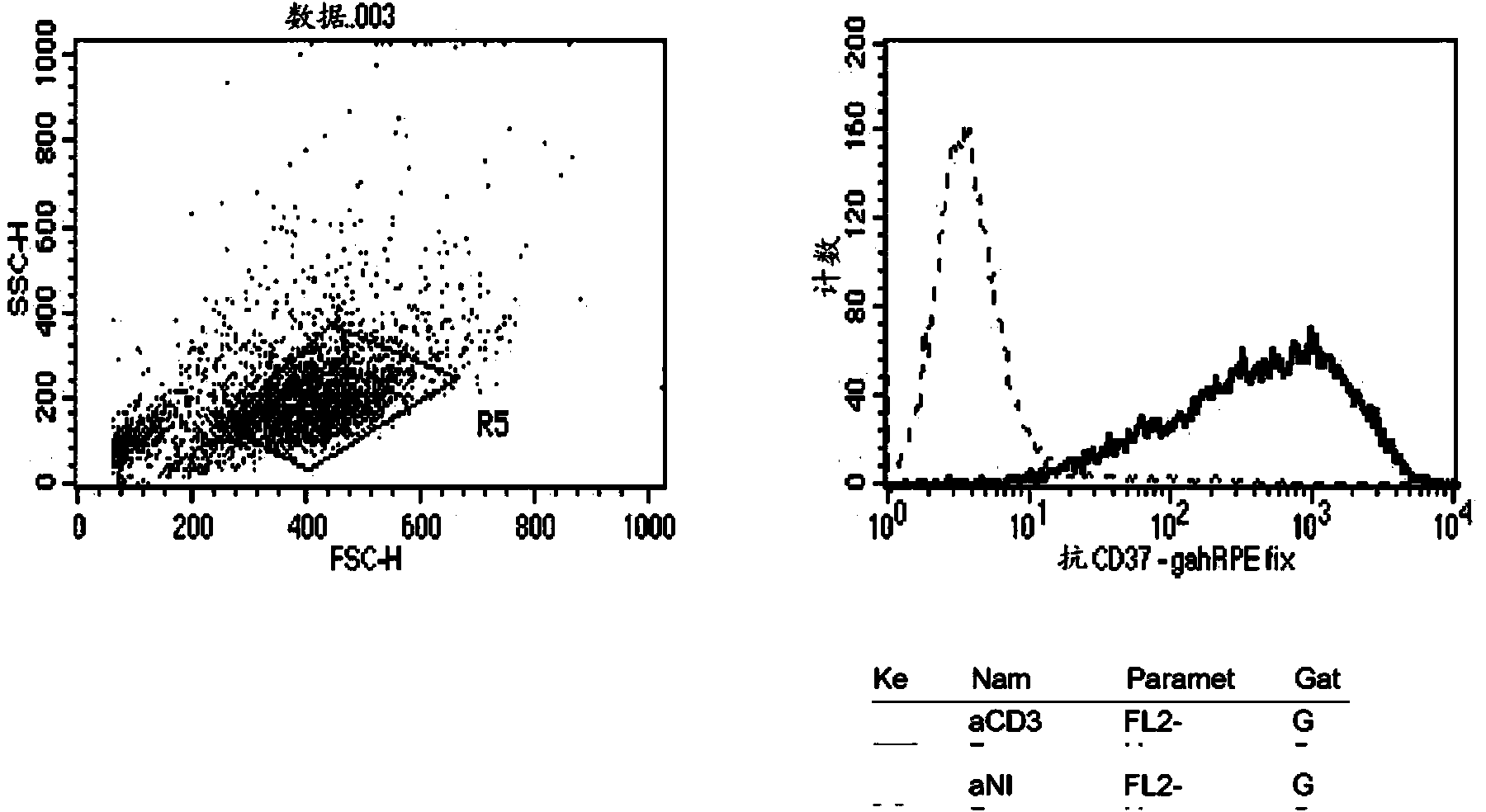
[0270] Example 2 Flow Cytometry for Assessing Antigen Binding
[0271] Binding analysis of hchIgG1ααCD37b(chHH1) to Daudi cells expressing CD37
[0272] Cells were stained and fixed.
[0273] The target binding of the constructed chHH1 was analyzed by flow cytometry. Daudi cells expressing CD37 were stained with chHH1 or hIgG1αNIP. In the left panel, intact Daudi cells were selected, and in the right panel the fluorescence histograms of these stained cells are shown. chHH1 bound to Daudi cells expressing CD37 (solid line), while chimeric αNIP IgG 1 No binding (dashed line).
Embodiment 3
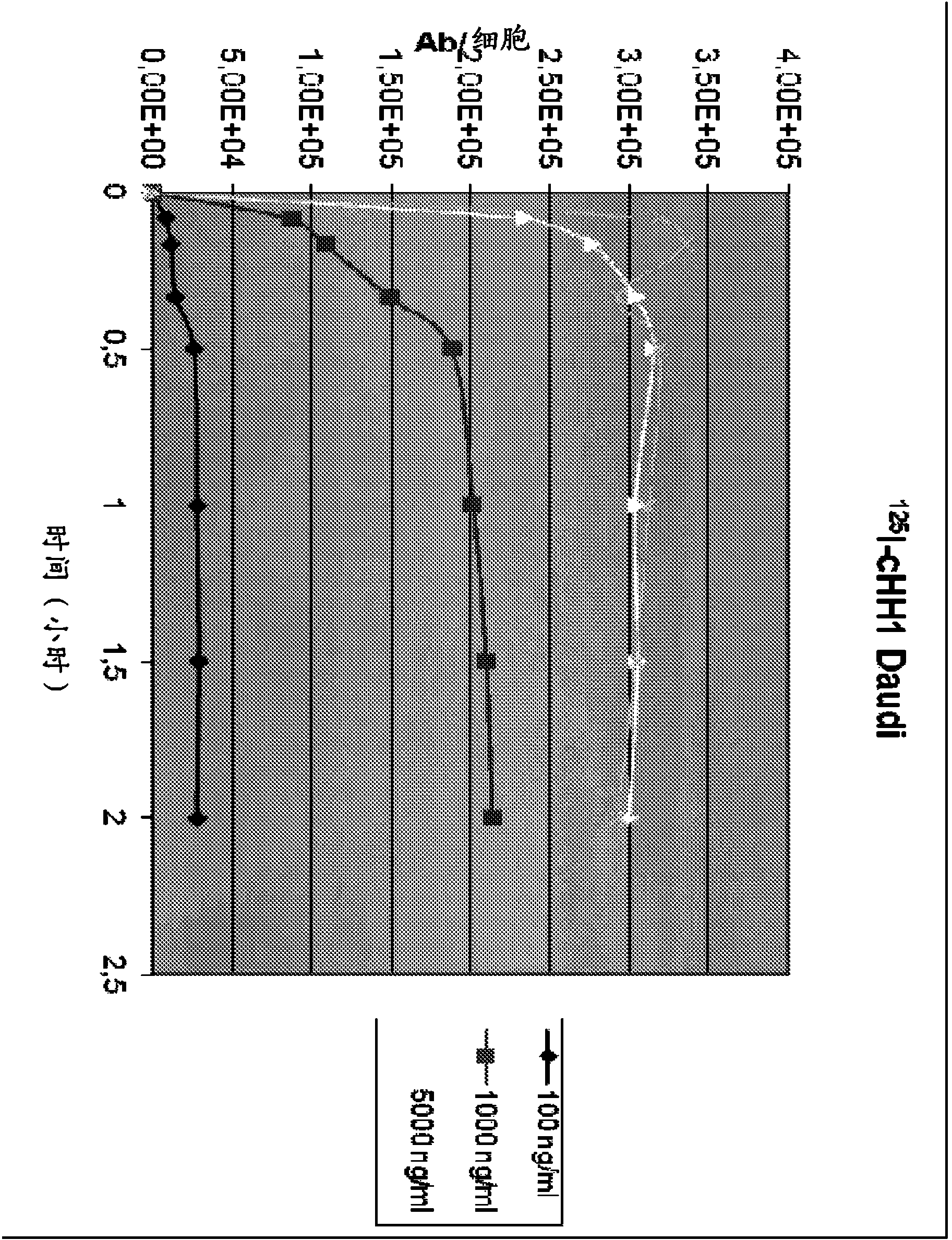
[0274] Example 3 Radiolabeling of Chimeric HH1
[0275] Iodination: According to the manufacturer's instructions, using IODOGEN pre-coated iodination test tubes (Pierce, Rockford, IL), by indirect iodination method with 125 I label the antibody.
[0276] use 111 In and 177 Labeling with Lu: First react the antibody with a chelator (p-SCN-Bn-DTPA or p-SCN-Bn-DOTA).
[0277] The DTPA or DOTA chelator was dissolved in 0.05M HCl, then added to the antibody, and the pH was adjusted to approximately 8.5 by washing with carbonate buffer at a ratio of 5:1. The pH is then checked again and adjusted if necessary. The solution was shaken at room temperature for 60 minutes, and then the reaction was stopped by adding 50 μl of 200 mM glycine solution (per mg antibody).
[0278] To remove free chelator, the conjugated antibody was washed 4-5 times with PBS (PAA), then adjusted to pH 5 by washing with ammonium acetate. followed by 111 In or 177 Lu (Perkin Elmer, Boston, Ma, USA) was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com