PPAR alpha/gamma dual agonist and its application
A dual agonist and compound technology, which is applied in the field of medicine, can solve the problem of single effect and achieve the effect of promoting fat cell differentiation, increasing mRNA level, and lowering blood sugar level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
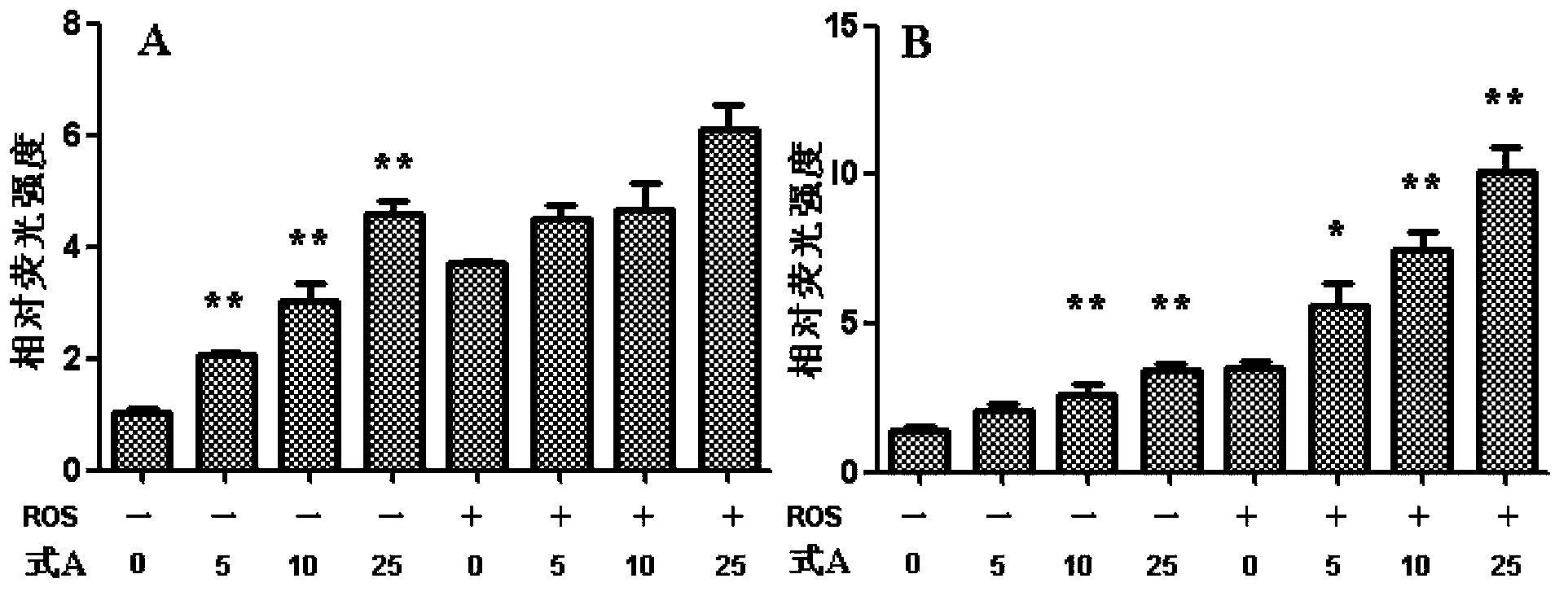
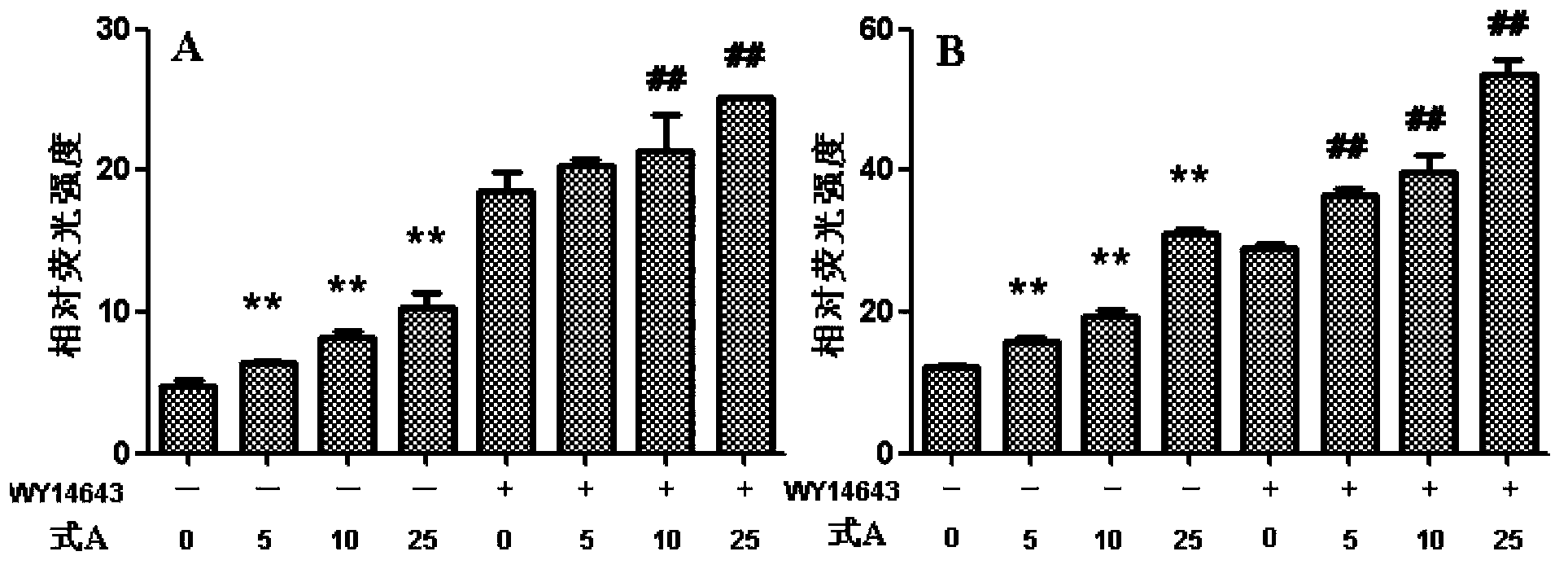
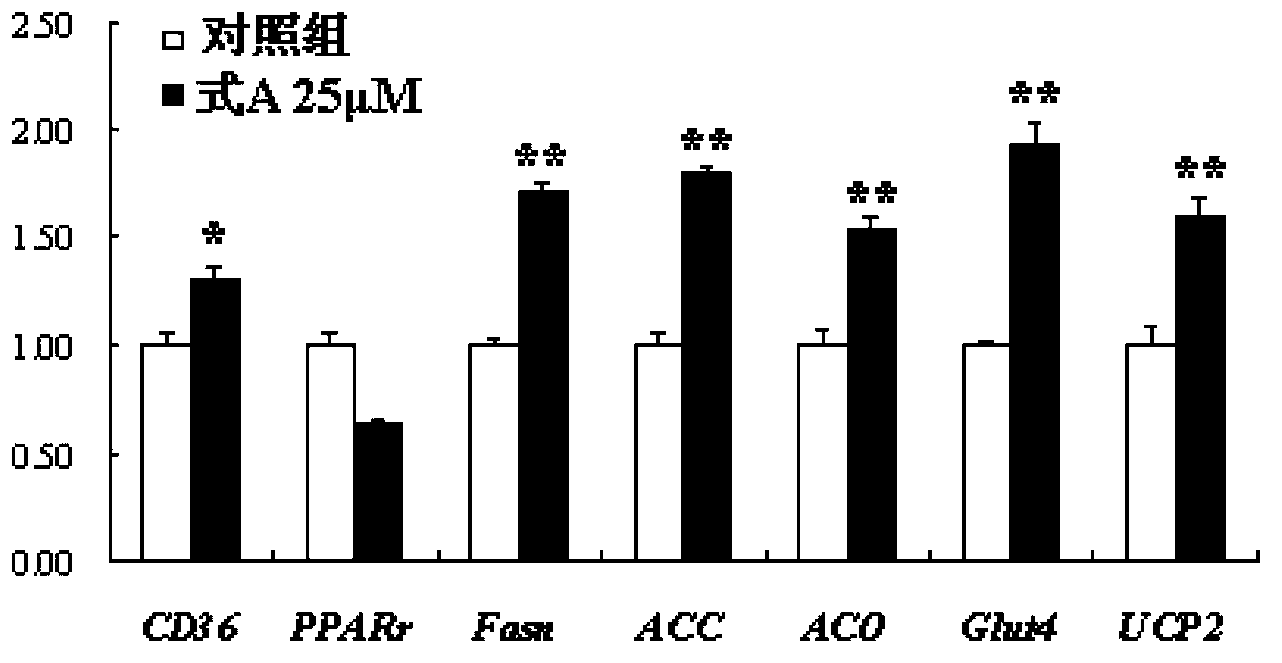
[0029] Example 1: Preparation of psoralen flavone methyl ether (compound of formula A)
[0030] 10.0kg of psoralen medicinal material was reflux extracted with 8 times the volume (80L) of ethanol aqueous solution with a volume fraction of 95%, refluxed for 2 hours each time, refluxed and extracted 3 times in total, combined extracts, concentrated under reduced pressure to obtain extract (approx. 800mL); add 1 times the amount (800mL) of water to the extract to suspend, then extract with petroleum ether (1000mL×3) and ethyl acetate (1000mL×3) in sequence, and collect the ethyl acetate extract; recover under reduced pressure Carry out silica gel column chromatography after ethyl acetate, and carry out gradient elution with petroleum ether and ethyl acetate successively (10:1-1:5); Apply the obtained fraction to silica gel column chromatography: use cyclohexane-acetone gradient first Elution (9:1-1:1), then use reverse column chromatography methanol-water gradient elution (60% me...
Embodiment 2
[0035] Embodiment 2: the preparation of formula B compound
[0036] Take 20mg of psoralen dihydroflavone [English name is bavachin, also isolated from psoralen herb, the method of obtaining it can be found in the literature Bioorg.Med.Chem.2004,12:4387-4392.] dissolved in 1mL of pyridine, and then Add 1 mL of acetic anhydride, let stand at room temperature for 24 hours, then add 10 mL of ethyl acetate solvent, back-extract with water, collect the organic phase, concentrate, and perform Sephadex LH-20 column chromatography to obtain the compound of formula B as a white powder.
[0037] 1 H-NMR (CDCl 3 ,400MHz)δ:1.72(3H,s,CH3-5''),1.77(3H,s,CH3-4''),2.34(6H,s,2×CH 3CO),2.88(1H,dd,J=2.8,16.8Hz,H-3),3.06(1H,dd,J=13.2,16.8Hz,H-3),3.23(2H,d,J=7.2Hz, H-1''),5.22(1H,m,H-2''),5.49(1H,dd,J=2.8,13.2Hz,H-2),6.80(1H,s,H-8),7.18 (2H,d,J=8.4Hz,H-3',5'),7.51(2H,d,J=8.4Hz,H-2',6'),7.82(1H,s,H-5).
Embodiment 3
[0038] Embodiment 3: the preparation of formula C compound
[0039] Dissolve 20mg of psoralen dihydroflavone methyl ether [compound of formula A] in 1mL of pyridine, then add 1mL of acetic anhydride, let stand at room temperature for 24h, then add 10mL of ethyl acetate solvent, back-extract with water, collect the organic phase, concentrate, Sephadex LH-20 column chromatography, the compound of formula C is obtained as white powder.
[0040] 1 H-NMR (CDCl 3 ,400MHz)δ:1.72(3H,s,CH3-5''),1.76(3H,s,CH3-4''),2.35(3H,s,CH3CO),2.83(1H,dd,J=2.8, 16.8Hz,H-3),3.04(1H,dd,J=13.2,16.8Hz,H-3),3.27(2H,d,J=7.2Hz,H-1''),3.88(3H,s, OCH3),5.29(1H,m,H-2''),5.47(1H,dd,J=2.8,13.2Hz,H-2),6.47(1H,s,H-8),7.18(2H,d ,J=8.4Hz,H-3',5'),7.52(2H,d,J=8.4Hz,H-2',6'),7.70(1H,s,H-5).
PUM
| Property | Measurement | Unit |
|---|---|---|
| humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com