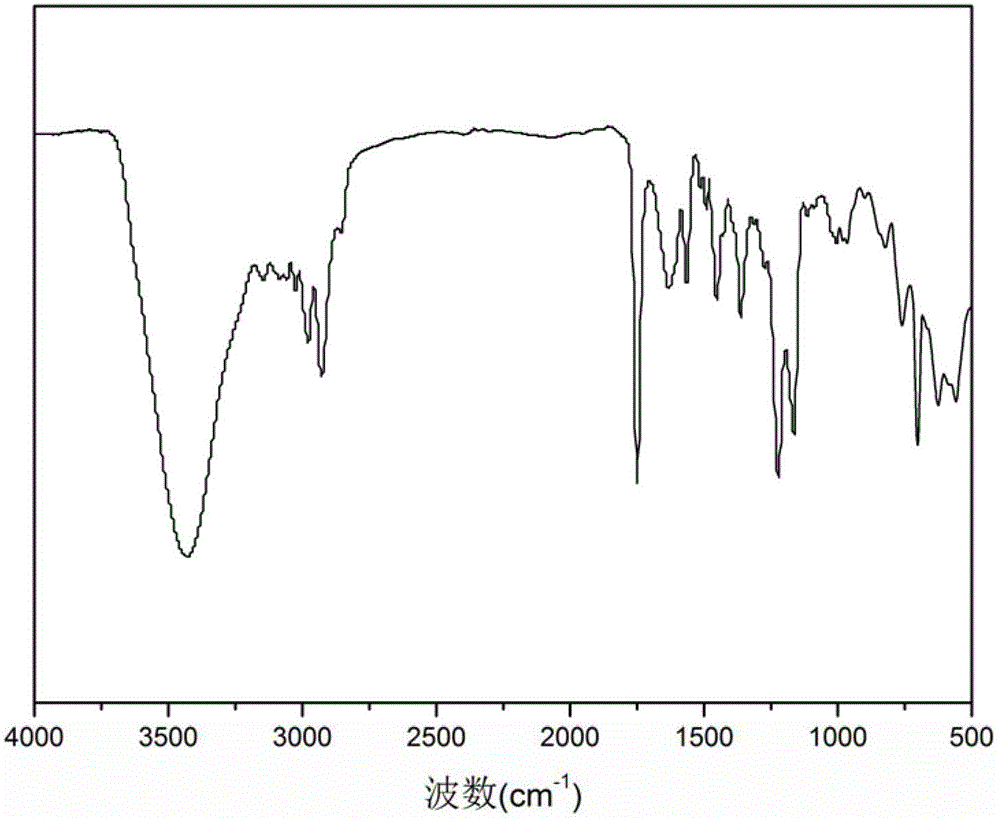
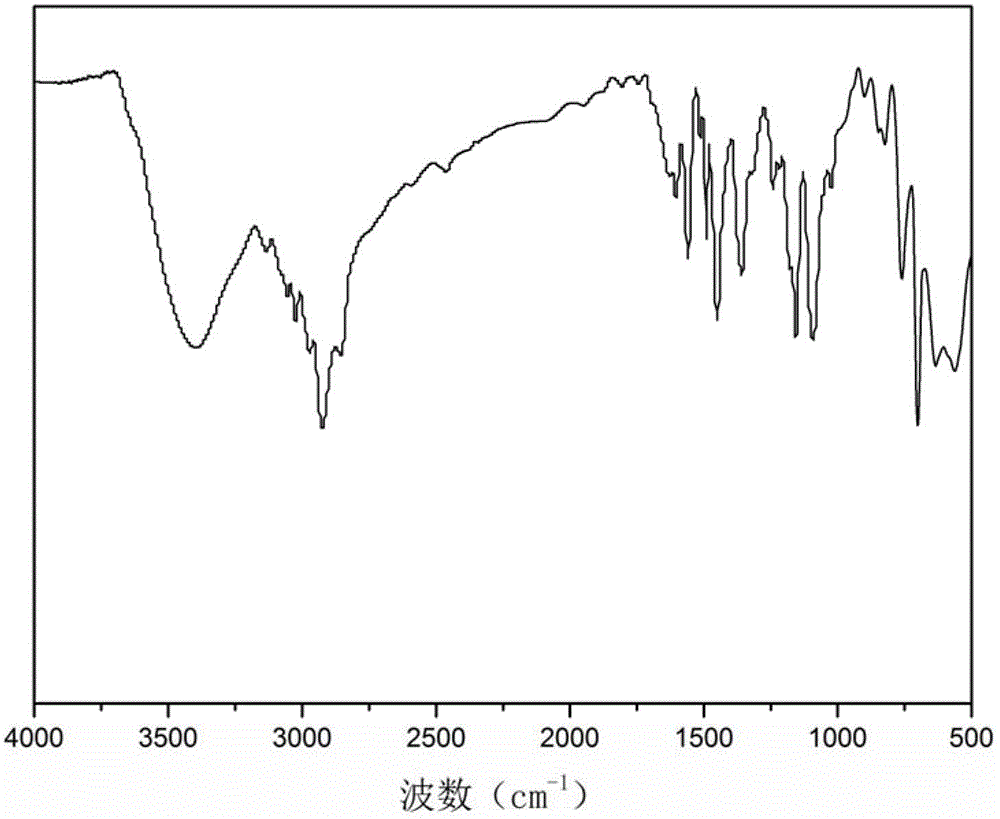
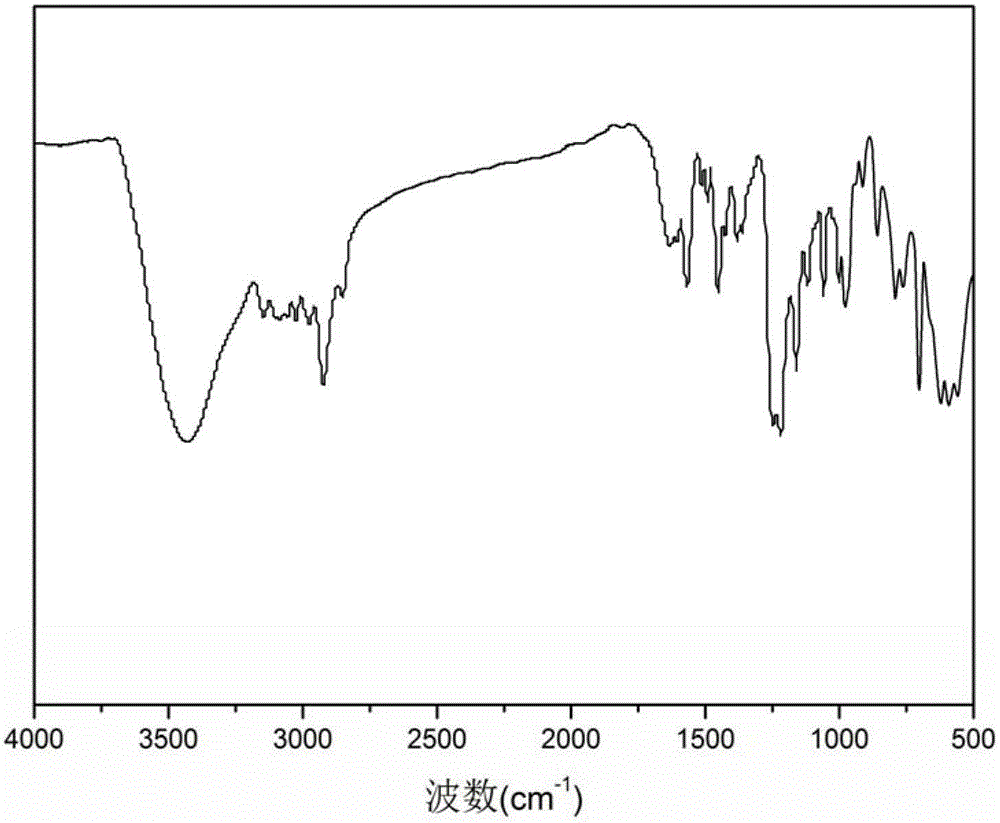
Nano magnetic microsphere supported tempo catalyst and its synthesis method and application
A nano-magnetic and synthetic method technology, applied in the direction of chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of increased reaction costs, instability of inorganic magnetic nanoparticles, Issues such as reunion and aggregation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] Preparation of Nanomagnetic Microsphere II:
[0055] FeCl 3 ·6H 2 O (24.4255 g, 0.09 mol) and FeSO 4 ·7H 2 O (16.7044g, 0.06mol) was added to 200ml of deionized water, heated to 90°C, and then 60ml of ammonia water, 3g of oleic acid, and 1g of undecylenic acid were added dropwise. After reacting for 3h, the precipitate was washed with deionized water to medium properties, to obtain nano-Fe 3 o 4 magnetic core.
[0056] 10g nanometer Fe 3 o 4 The magnetic core is mixed with 10g styrene, 10g p-chloromethylstyrene, 1g divinylbenzene and 2g hexadecane to form an oil phase, then 300ml deionized water and 3g sodium lauryl sulfate are mixed to form a water phase, and the Add the above oil phase to the water phase dropwise under the conditions to obtain a black suspension; place the black suspension in an ice-water bath, use a 300W cell pulverizer to finely emulsify it for 30 minutes, pour the fine emulsion into a reaction vessel, and add 0.1g K 2 S 2 o 8 , N 2 Stir...
Embodiment 1
[0062] Put 4-hydroxy TEMPO (5mmol), bromoacetic acid (5mmol), dichloromethane (25ml) in a round bottom flask, N 2 Stir in an ice-water bath under protection, then add DCC (dicyclohexylcarbodiimide, 6mmol), DMAP (4-dimethylaminopyridine, 0.05mmol) dropwise into the reaction system, and stir at room temperature for 12h; The product is washed and dried to obtain a red product;
[0063] Put magnetic microsphere II (containing 5mmol of Cl), imidazole (10mmol), and appropriate amount of benzene in a round bottom flask, N 2 Under protection, stir and react in an oil bath at 80°C for 24 hours, wash with acetone, filter, extract, and dry to obtain the magnetic microspheres grafted with imidazole III;
[0064] The above red product (5 mmol), imidazole-loaded magnetic microspheres III (containing 2.5 mmol of imidazole groups) were added to the reactor, benzene was used as solvent, and N 2 Stir the reaction in an oil bath at 80°C for 12 hours under protection, wash and dry the reaction ...
Embodiment 2
[0068] 4-Hydroxy TEMPO (2.58g, 0.015mol) was dissolved in acetone (25ml), NaH (0.6g, 0.015mol) was added, the mixture was stirred at room temperature for 30min, and 1,4-dibromobutane (4.86g , 0.0225mol), stirred at room temperature for 3h, after the reaction was over, the acetone was distilled off under reduced pressure, and the resulting solid was dissolved in distilled water; the aqueous layer was extracted three times with dichloromethane, and then Na 2 SO 4 Dry the extract containing organic matter, and then vacuum dry to obtain a concentrated solution; use a chromatographic column (petroleum ether / ethyl acetate = 9:1) to separate and dry the concentrated solution;
[0069] Then the first step reaction product (5mmol), imidazole-loaded magnetic microsphere III (containing imidazole group 5mmol) prepared according to the method of Example 1, K 2 CO 3 Add (5mmol) into a round bottom flask, use acetone as a solvent, stir and react at 80°C for 6h; cool to room temperature, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com