Preparation method of cardanol-base polyalcohols and polyurethanes thereof
A cardanol-based and cardanol-based technology, which is applied in the preparation of two cardanol-based polyols and polyurethanes, can solve the problems of lack of hardness, coating wear resistance and poor chemical resistance, and achieve rich sources and excellent flexibility Good effect of sex and hardness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In a 100ml round bottom flask, add 20g cardanol, 23.5g 2-mercaptoethanol, 0.87g 2-hydroxy-2-methyl-1-phenyl-1-acetone and 50ml dichloromethane. Reaction for 10 hours under light irradiation, wash with water 5-6 times and then use anhydrous MgSO 4 After drying, the cardanol-based polyol is removed from the solvent, and the number of hydroxyl groups is 2 by nuclear magnetic measurement.
Embodiment 2
[0026] In a 100ml round bottom flask, add 20g cardanol, 23.5g 2-mercaptoethanol, 0.87g 2-hydroxy-2-methyl-1-phenyl-1-acetone and 50ml dichloromethane. React for 20 hours under light irradiation, wash with water 5-6 times and use anhydrous MgSO 4 After drying, the cardanol-based polyol is removed from the solvent, and the number of hydroxyl groups is 2.5 by nuclear magnetic field measurement.
Embodiment 3
[0028] In a 100ml round bottom flask, add 20g cardanol, 23.5g 2-mercaptoethanol, 0.87g 2-hydroxy-2-methyl-1-phenyl-1-acetone and 50ml dichloromethane. React under light irradiation for 45 hours, wash with water 5-6 times and use anhydrous MgSO 4 After drying and removing the solvent, a cardanol-based polyol was obtained, and the number of hydroxyl groups was 3.3 by nuclear magnetic measurement.
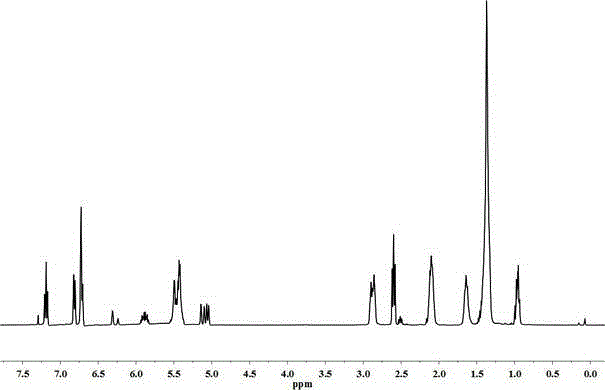
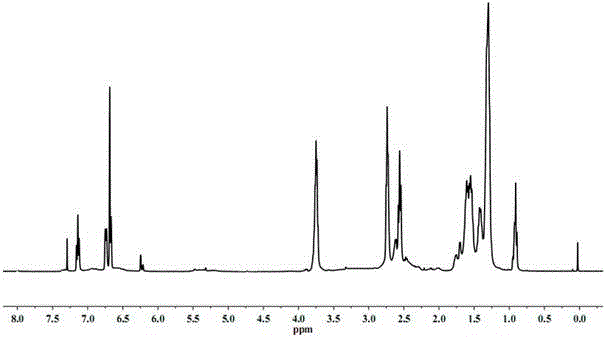
[0029] Cardanol 1 HNMR chart see figure 1 , Cardanol polyol (3.3 hydroxyl groups) 1 HNMR chart see figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com