Kit based on serum miRNA as well as use method and application of kit
A kit and serum technology, applied in the field of serum miRNA-based kits, can solve problems such as difficulty in laboratory development, poor sensitivity, and dependence on viremia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: serum total RNA is extracted
[0016] Collect 6 serum samples from patients with acute SFTS and 6 healthy normal serum samples, mix the two groups of serum (200μL each), and extract total RNA using the phenol-chloroform method. Specific steps: 1. Take 100ul serum samples and add 300ul DEPC water 1.5ml EP, then add 1ul10f / ul cel-miR-238 for parameter calibration, mix well, add 200ul water-saturated phenol, shake vigorously, let stand for 3min, add 200ul chloroform, shake well again and mix 16000g Centrifuge for 15 minutes at room temperature. 2. Aspirate the supernatant, add isopropanol twice the volume of the supernatant, then add 1 / 10 of the volume of the supernatant 3M sodium acetate about 40ul, mix well, let stand at -20°C for 2h, and then store at 4°C, 16000g , Centrifuge for 20min. 3. Discard the supernatant, add 1ml of 75% ethanol, centrifuge at 16000g at 4°C for 20min. 4. Discard the supernatant, dry at room temperature, add 20ul DEPC water to di...
Embodiment 2
[0017] Example 2: Detection of differential expression of serum miRNA in SFTS acute infection
[0018] TaqMan Array Human v3.0 chip (Applied Biosystems, USA) was used for detection. For each group of samples, 212 miRNAs were detected on the Array A plate, and U6snRNA was selected as the internal reference. 1. Reverse transcription using Taqman MiRNA Reverse Transcription Kit (Applied Biosystems, USA): add 50ng total RNA to 4.5ul Megaplex pool reverse transcription mixture, including Megaplex RT Primers (10×) 0.8μl, dNTPs 0.2μl, MultiScribe Reverse Transcriptase (50U / μl) 1.5μl, RT Buffer (10×) 0.8μl, MgCl2 (25mM) 0.9μl, RNase inhibitor (20U / μl) 0.1μl, Nuclease-free water 0.2μl. 2. Pre-amplification: TaqMan PreAmp Mastermix (2×) 12.5 μl and Megaplex PreAmp Primer (10×) 2.5 μl, reverse transcription product 2.5 μl, Nuclease-free water 7.5 μl. 3. The qRT-PCR reaction was carried out on a 7900HT instrument (Applied Biosystems): dilute the pre-amplification reaction solution 4 tim...
Embodiment 3
[0020] Example 3: Verification of serum differentially expressed miRNA
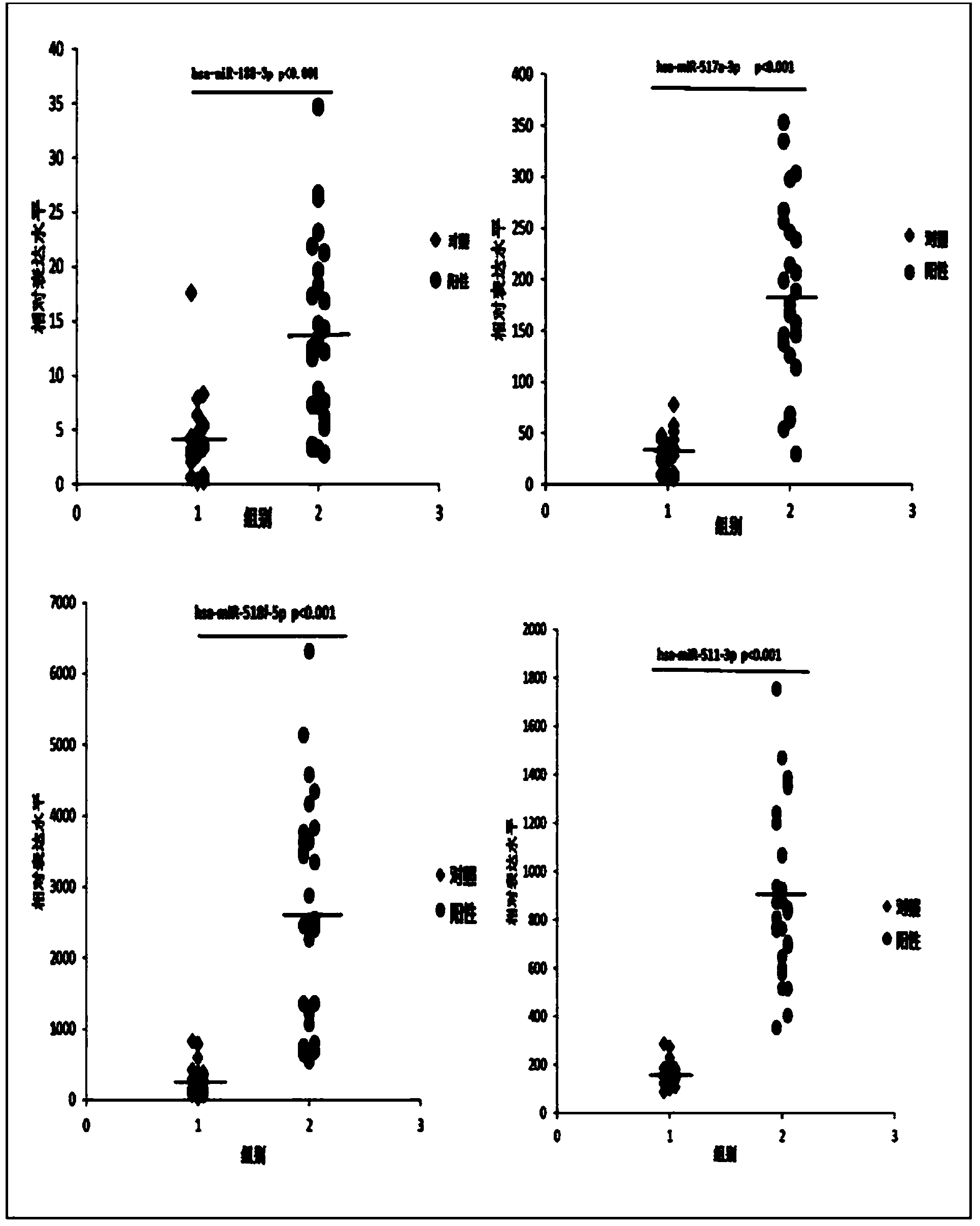
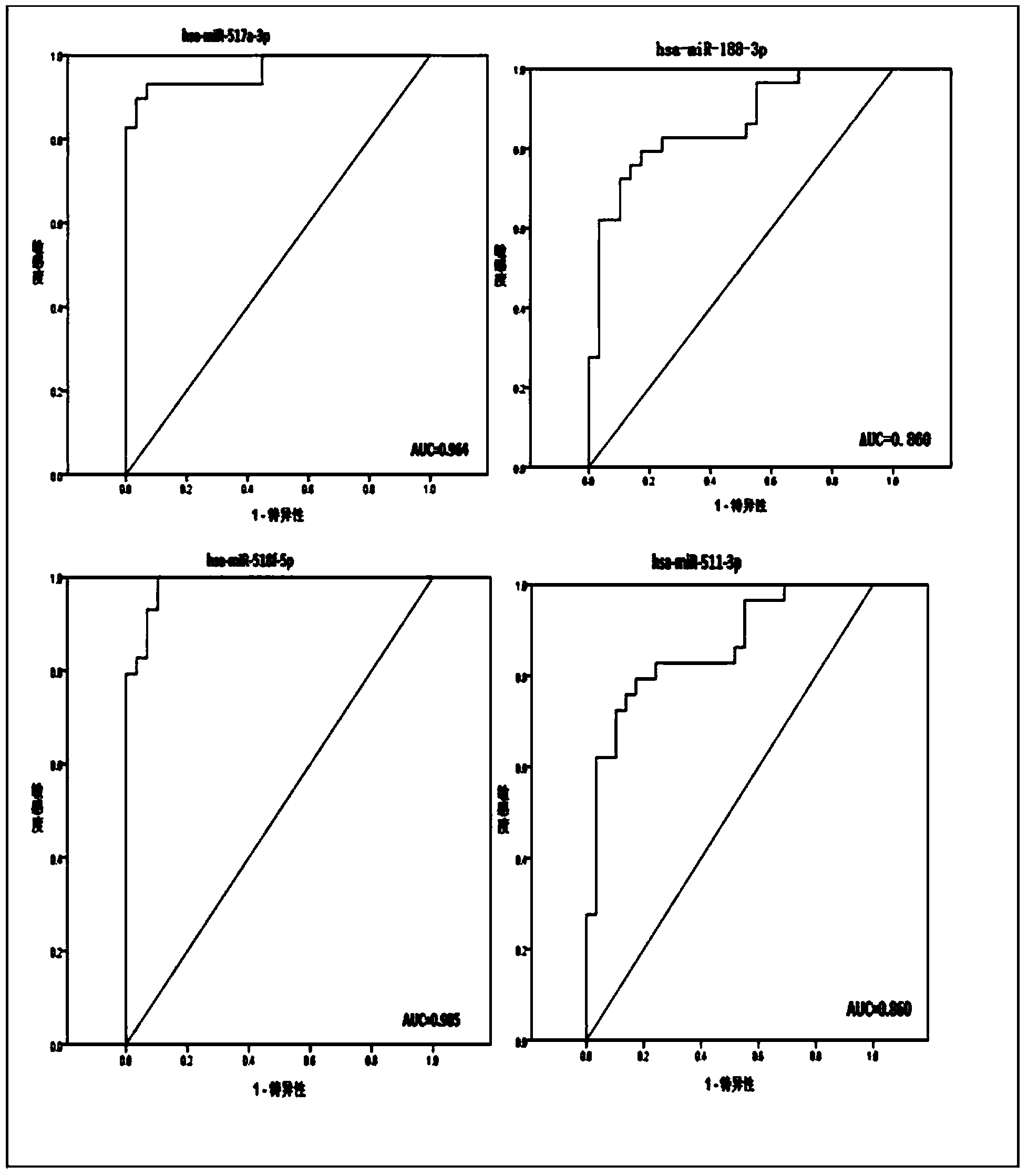
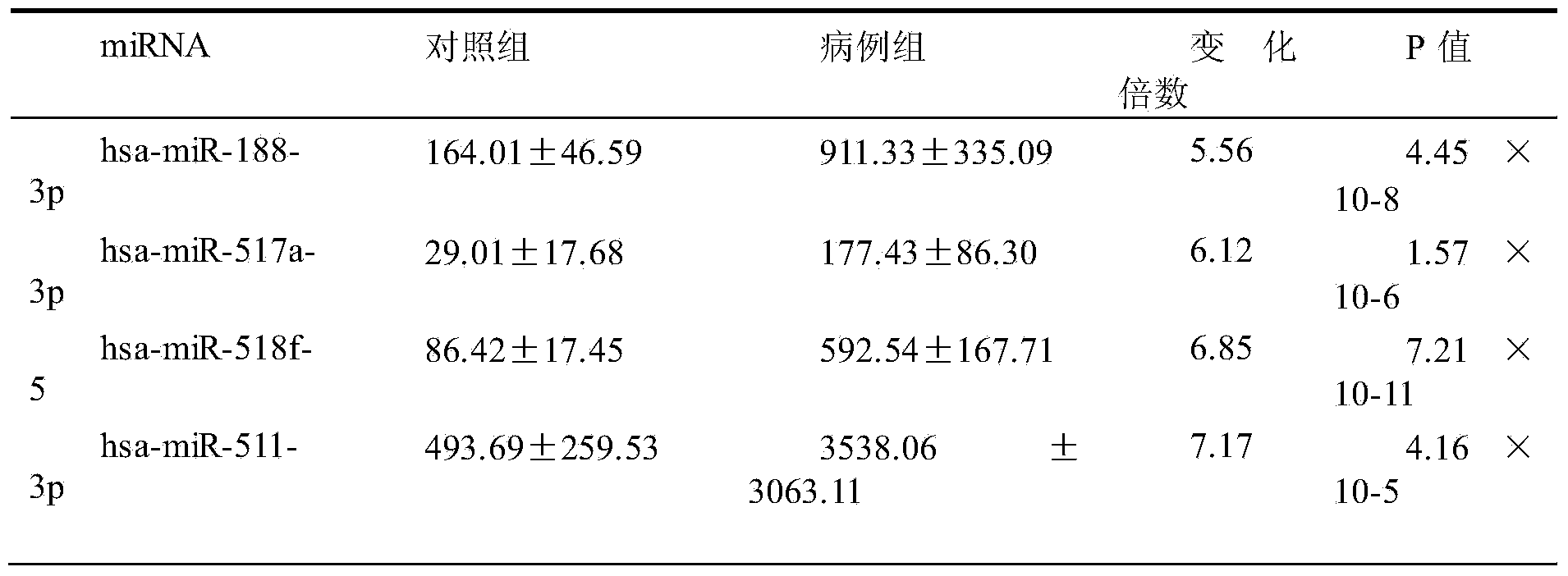
[0021] The 30 miRNAs specifically expressed in the sera of SFTS acutely infected patients obtained in Example 2 were verified by qRT-PCR. 58 sera were selected for the verification experiment, 29 of which were from the acute phase of SFTS infection, and 29 were from healthy people. 30 miRNA-specific primers and probes were designed and synthesized by Applied Biosystems in the United States, and artificially synthesized single-stranded miR-16 was used as an internal reference gene standard. Each sample of 22 miRNAs was detected in triplicate, and the differential expression level was calculated by 2-(ΔCtmiRNA-ΔCtcontrol). In this experiment, SPSS18.0 software was used for statistical analysis, and the data representation method was median ± SD. The comparison between groups was performed by t test, and P<0.05 was considered significant difference.
[0022] Table 1 Comparison of serum miRNA expression dif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com