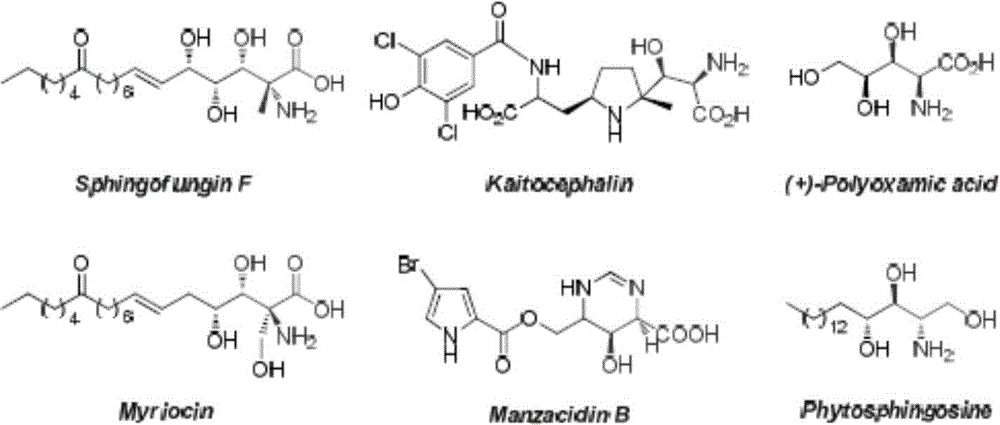
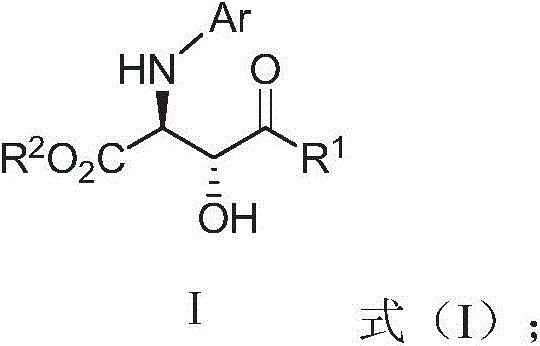
Optically active α-amino β-hydroxyamino acid derivatives and preparation method and application thereof
A hydroxyamino acid and optically active technology, which is applied in the field of α-amino-β-hydroxyamino acid derivatives and their preparation, can solve problems such as multi-step operations and complex reaction substrates, improve the utilization rate of raw materials, and simplify the synthesis route , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Weigh N-p-chlorophenyl glycine ethyl ester (0.20mmol), rhodium acetate (0.002mmol), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (0.20mmol) and chiral Phosphoric Acid A (0.01 mmol) was put into a small test tube reactor, 2.0 ml of 1,2-dichloroethane was added, stirred at room temperature for 4 hours, and then placed at -10°C. Weigh acetophenone diazonium (0.30mmol) and dissolve it in 1.0ml 1,2-dichloroethane, and inject it into the reaction system through a peristaltic pump for 1 hour. After the injection is completed, continue to stir overnight, and remove it by rotary evaporation at 50°C-60°C. Solvent, to obtain crude product; then separated by column chromatography (eluent: petroleum ether: ethyl acetate = 1:20 ~ 1:5) to obtain pure optically active α-amino-β-hydroxy amino acid derivatives 2A. The yield was 80%, the dr value was 95:5, and the ee was 93%.
[0040] In this example, chiral phosphoric acid A is: Structural formula
[0041] Product 2A is: structural form...
Embodiment 2
[0045] Weigh N-p-chlorophenyl glycine ethyl ester (0.20mmol), rhodium acetate (0.002mmol), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (0.20mmol) and chiral Phosphoric Acid A (0.01 mmol) was put into a small test tube reactor, 2.0 ml of 1,2-dichloroethane was added, stirred at room temperature for 4 hours, and then placed at -10°C. Weigh p-methylacetophenone diazo (0.30mmol) and dissolve it in 1.0ml 1,2-dichloroethane, and inject it into the reaction system through a peristaltic pump for 1 hour, and continue stirring overnight after the injection is completed, at 50°C-60°C The solvent was removed by rotary evaporation to obtain a crude product; then separated by column chromatography (eluent: petroleum ether: ethyl acetate = 1:20 ~ 1:5) to obtain an optically active α-amino-β-hydroxy amino acid derivative Pure product 2B. The yield was 85%, the dr value was 90:10, and the ee was 92%.
[0046] In this example, chiral phosphoric acid A is: Structural formula
[0047] Product ...
Embodiment 3
[0052] Weigh N-p-chlorophenyl glycine ethyl ester (0.20mmol), rhodium acetate (0.002mmol), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (0.20mmol) and chiral Phosphoric Acid A (0.01 mmol) was put into a small test tube reactor, 2.0 ml of 1,2-dichloroethane was added, stirred at room temperature for 4 hours, and then placed at -10°C. Weigh p-methoxyacetophenone diazonium (0.30mmol) and dissolve it in 1.0ml 1,2-dichloroethane, and inject it into the reaction system through a peristaltic pump for 1 hour. After the injection is completed, continue to stir overnight. The solvent was removed by rotary evaporation at ℃ to obtain the crude product; then separated by column chromatography (eluent: petroleum ether: ethyl acetate = 1:20 ~ 1:5) to obtain an optically active α-amino-β-hydroxy amino acid Derivative pure 2C. The yield was 74%, the dr value was 85:15, and the ee was 94%.
[0053] In this example, chiral phosphoric acid A is: Structural formula
[0054] Product 2C is: struct...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com