Preparation method of 1, 4-dihydropyridine compound
A technology for dihydropyridine and compound, which is applied in the field of preparation of 1,4-dihydropyridine compounds, can solve problems such as environmental pollution, and achieve the effects of no environmental pollution, easy recycling and simple reaction operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
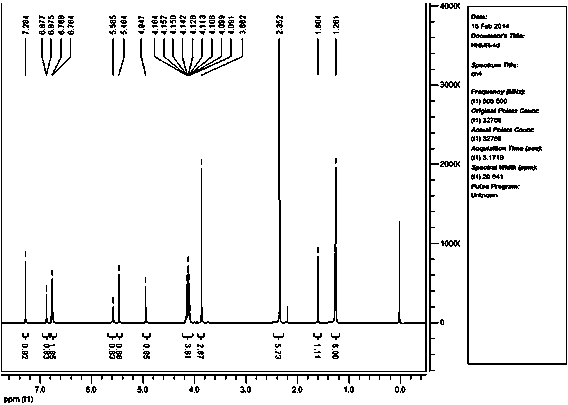
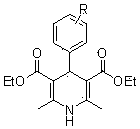
[0028] A preparation method of 1,4-dihydropyridine compounds, wherein the structural formula of the 1,4-dihydropyridine compounds is as follows:
[0029]
[0030] Wherein substituent R is 3-OCH 3 -4-OH group;
[0031] A kind of above-mentioned preparation method of 1,4-dihydropyridine compounds, the reaction equation of its synthetic process is as follows:
[0032] That is to say, aromatic aldehyde, ethyl acetoacetate and ammonium acetate are reacted at a temperature of 80°C for 3-4 hours in the presence of the catalyst, the ionic liquid SiPIPPWO. After the reaction, cool to room temperature, add ethyl acetate, and filter to remove the catalyst, the ionic liquid SiPIPPWO. , the filtrate was rotary evaporated to remove the solvent to obtain a crude product, and then recrystallized with 95% ethanol to obtain 1,4-dihydropyridine compounds;
[0033] The aromatic aldehyde is 3-methoxy-4-hydroxybenzaldehyde;
[0034] The aromatic aldehyde, ethyl acetoacetate, and ammonium a...
Embodiment 2
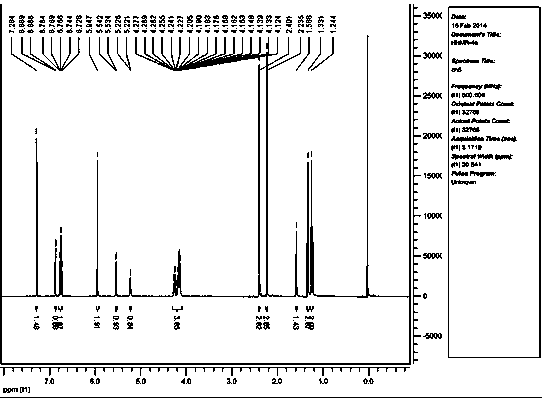
[0048] A kind of preparation method of 1,4-dihydropyridine compound, the structural formula of described 1,4-dihydropyridine compound is as follows:
[0049]
[0050] Wherein the substituent R is 3,4-OCH 2 O base;
[0051] A kind of above-mentioned preparation method of 1,4-dihydropyridine compounds, the reaction equation of its synthetic process is as follows:
[0052] That is to say, aromatic aldehyde, ethyl acetoacetate and ammonium acetate are reacted at a temperature of 80°C for 3-4 hours in the presence of the catalyst, the ionic liquid SiPIPPWO. After the reaction, cool to room temperature, add ethyl acetate, and filter to remove the catalyst, the ionic liquid SiPIPPWO. , the filtrate was rotary evaporated to remove the solvent to obtain a crude product, and then recrystallized with 95% ethanol to obtain 1,4-dihydropyridine compounds;
[0053] Described aromatic aldehyde is 3,4-dioxymethylene benzaldehyde;
[0054] The aromatic aldehyde, ethyl acetoacetate, and a...
Embodiment 3
[0067] A kind of preparation method of 1,4-dihydropyridine compound, the structural formula of described 1,4-dihydropyridine compound is as follows:
[0068]
[0069] Wherein substituent R is -H group;
[0070] A kind of above-mentioned preparation method of 1,4-dihydropyridine compounds, the reaction equation of its synthetic process is as follows:
[0071] That is to say, aromatic aldehyde, ethyl acetoacetate and ammonium acetate are reacted at a temperature of 80°C for 3-4 hours in the presence of the catalyst, the ionic liquid SiPIPPWO. After the reaction, cool to room temperature, add ethyl acetate, and filter to remove the catalyst, the ionic liquid SiPIPPWO. , the filtrate was rotary evaporated to remove the solvent to obtain a crude product, and then recrystallized with 95% ethanol to obtain 1,4-dihydropyridine compounds;
[0072] Described aromatic aldehyde is benzaldehyde;
[0073] The aromatic aldehyde, ethyl acetoacetate, and ammonium acetate used in the ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com