A kind of preparation method of diphtheria vaccine
A diphtheria vaccine and diphtheria bacillus technology, applied in the field of vaccines, can solve the problems of long production cycle of diphtheria vaccine, excessive impurity protein in the final product, and large side reactions of the product, so as to reduce the risk of pollution, low impurity protein content, and high recovery rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
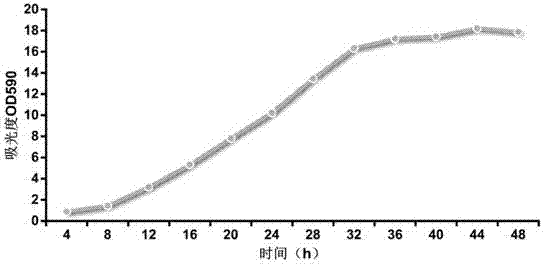
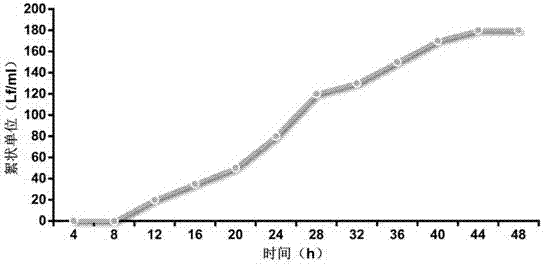
[0022] like figure 1 Shown, a kind of preparation method of diphtheria vaccine is characterized in that, comprises the following steps:
[0023] 1) resuscitating, fermenting and culturing diphtheria bacteria strains to prepare diphtheria bacteria liquid;
[0024] 2) centrifuging the diphtheria bacterium liquid, filtering it three times, then performing ultrafiltration concentration, dialysis and washing, thereby obtaining diphtheria toxin;
[0025] 3) Detoxify the diphtheria toxin, and then use a tangential flow ultrafiltration system to perform ultrafiltration concentration, dialysis and washing;
[0026] 4) Purifying the detoxified diphtheria toxin.
[0027] As a preferred technical solution, the specific method of step 2) is: filter the diphtheria bacteria solution after centrifugation through a 0.22 μm → 0.1 μm filter membrane, then filter through a 300KD hollow fiber column, and then use a 10KD hollow fiber column to perform Concentrate by ultrafiltration, and when the...
specific Embodiment
[0032] Experiment 1) Diphtheria bacteria fermentation culture
[0033] Experimental Materials:
[0034] Strains: Bacillus diphtheriae PW8 strain (CMCC38007)
[0035] Medium: Improved synthetic medium: acid hydrolyzed casein 20g / L, peptone 5g / L, maltose 2.5g / L, lactic acid 2.5ml / L, Mueller's No. I solution 1ml / L, II solution 2ml / L ; The iron ion concentration of the culture medium is lower than 0.10mg / ml.
[0036] Rehydration: 20% maltose, Mueller's I, II solution, 1% glutamic acid solution.
[0037] Main equipment: 50L fermentation tank (Shanghai Wanmuqing Biological Engineering Co., Ltd.)
[0038] experimental method:
[0039] After the recovery of diphtheria strains, cultivate them on the improved liquid medium at 37°C for 72 hours, then scrape the bacterial film and spread it on the liquid medium to shake the flask, and cultivate at 37°C for 48 hours. Prepare 30L of liquid culture medium according to 20g / L of acid hydrolyzed casein, 5g / L of peptone, 2.5g / L of maltose, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com