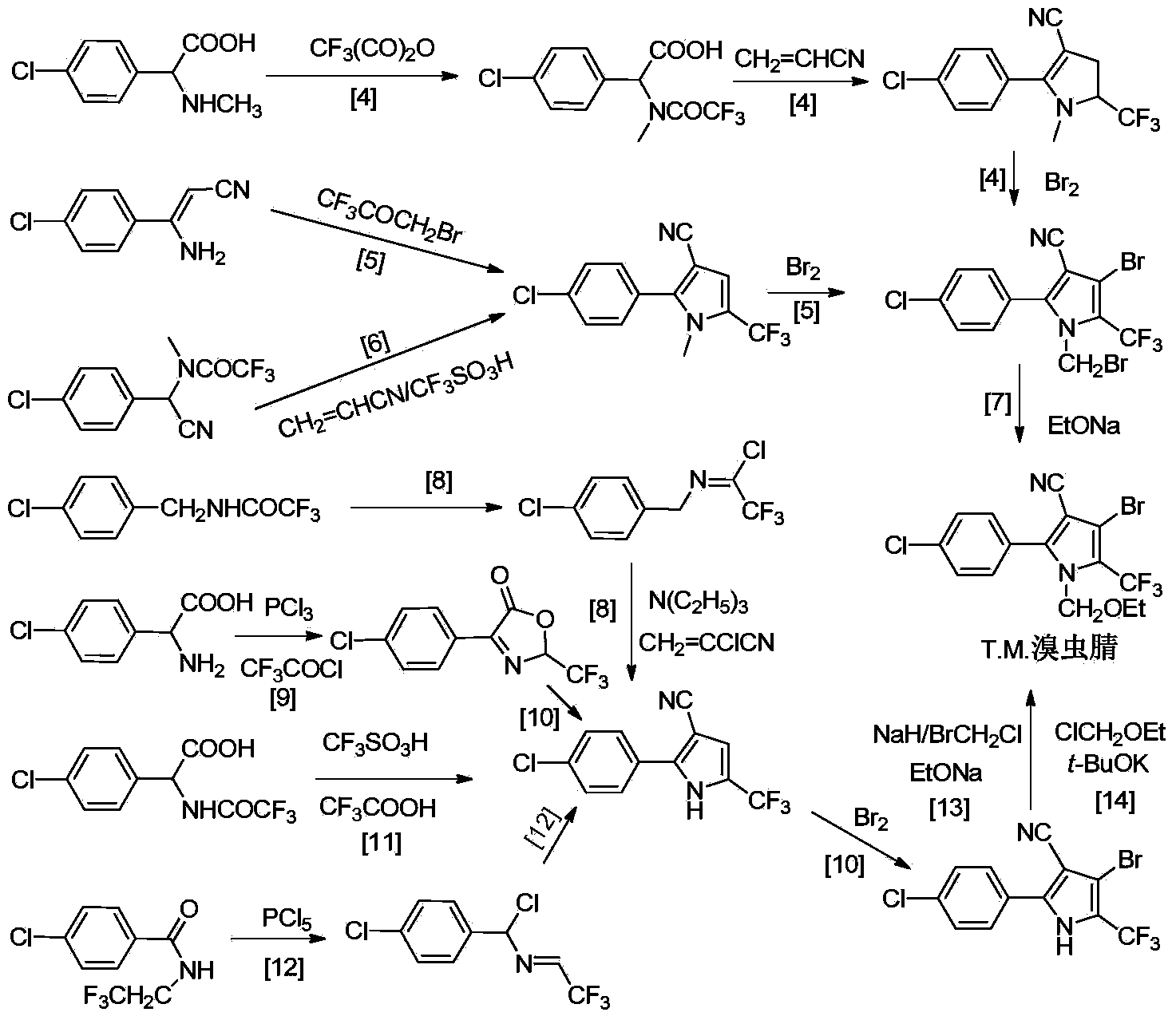
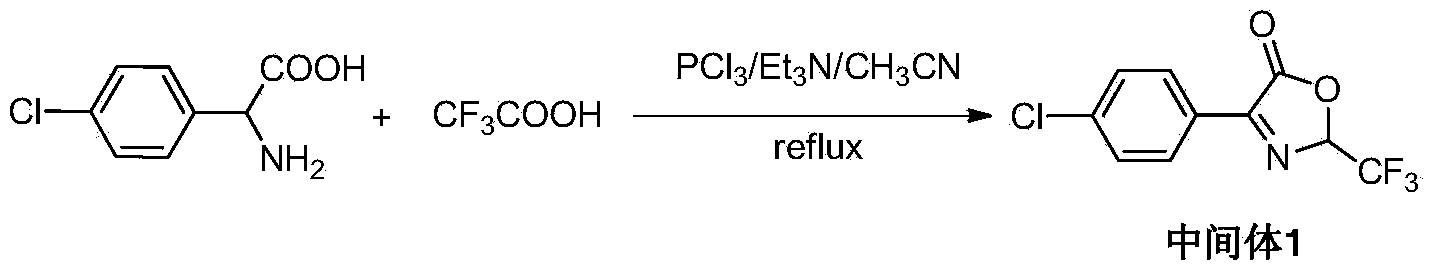Synthetic method for chlorfenapyr
A synthetic method, the technology of chlorfenapyr, which is applied in the synthetic field of chlorfenapyr-1-ethoxymethyl-5--pyrrole-3-carbonitrile), can solve the problem of high raw material cost and highly toxic chemical sodium cyanide , Restricting industrial development and marketing, and poor selectivity of the reaction, etc., to achieve the effects of reducing raw material costs and safety risks, easy operation, and mild reaction conditions in the synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the synthesis of intermediate 4-(p-chlorophenyl)-2-trifluoromethyl-3-oxazoline-5-ketone body
[0027]
[0028] Add 50 mL of acetonitrile to a 250 mL round bottom flask, add 5.0 g (0.025 mol, 1.0 eq) of p-chlorophenylglycine, stir evenly with a magnetic force, and add 2.9 mL (0.0375 mol, 1.5 eq) of trifluoroacetic acid dropwise for about 5 min. After mixing evenly, slowly add catalyst triethylamine 3.5mL (0.025mol, 1.0eq) dropwise, then slowly add phosphorus trichloride 2.4mL (0.0275mol, 1.1eq), dropwise within 0.5h. Finally, the temperature was raised to 65°C and the reaction was maintained for 4h. After the reaction was complete, it was cooled to room temperature, concentrated by rotary evaporation, and an appropriate amount of toluene was added to spin dry. A yellow viscous oily product was obtained, namely intermediate 1—(4-(p-chlorophenyl)-2-trifluoromethyl-3-oxazolin-5-one).
Embodiment 2
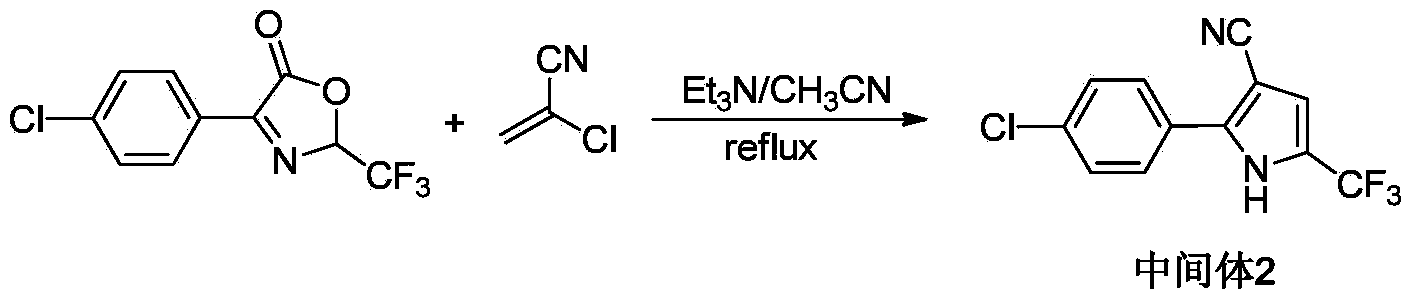
[0029] Embodiment 2: the synthesis of intermediate 2-(p-chlorophenyl)-5-(trifluoromethyl)-pyrrole-3-carbonitrile
[0030]
[0031]The 250mL round bottom flask used in the first step reaction was directly used for the next step reaction, and 50mL of acetonitrile was added, wherein the amount of intermediate 1 was about 5.3g (0.02mol, 1.0eq). After stirring at room temperature, 3.5 g (0.04 mol, 2.0 eq) of 2-chloroacrylonitrile was added. A quantitative catalyst triethylamine 7.2mL (0.05mol, 2.5eq) was added dropwise, and the dropping rate was controlled at 6-7min / mL. After the dropwise addition, reflux at about 78°C for 1h. After the reaction is complete, cool to room temperature, add an appropriate amount of cold water to the reaction solution, a light yellow solid precipitates, and filter under reduced pressure to obtain the product, the intermediate 2—(2-(p-chlorophenyl)-5-(trifluoromethane base)-pyrrole-3-carbonitrile), and the product was recrystallized from ethanol. ...
Embodiment 3
[0033] Embodiment 3: the synthesis of intermediate 4-bromo 2-(p-chlorophenyl)-5-(trifluoromethyl)-pyrrole-3-carbonitrile
[0034]
[0035] Add 75.0mL of acetic acid into a 250mL two-necked flask, add 25.4g (0.020mol, 1.0eq) of the intermediate, stir evenly with a magnetic force, add 1.97g (0.024mol, 1.2eq) of anhydrous sodium acetate, and after completely dissolving, slowly heat to 90.0°C, stir for 10 minutes. At a constant temperature of 90.0°C, 20.0 mL of acetic acid solution of bromine (6.4 g, 0.040 mol, 2.0 eq) was added dropwise, and the rate of addition was adjusted according to whether there was orange-red bromine vapor in the condenser tube. After the dropwise addition was completed, the stirring was continued for 30 minutes, then the temperature was raised to 110.0° C., and the reaction was maintained for 3.0 hours. After the reaction was complete, the system was cooled to room temperature, and an equal amount of ice water was added, and a large amount of white so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com