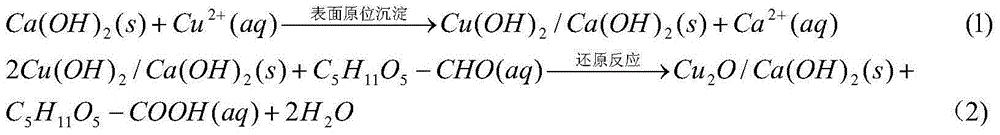A method for preparing cu2o/ca(oh)2 nanocomposite photocatalyst by interface reduction method
A nanocomposite and photocatalyst technology, applied in nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, can solve problems such as complex reaction process, environmental pollution, and easy agglomeration of nanoparticles, so as to achieve easy separation Recycling, overcoming separation and recycling, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
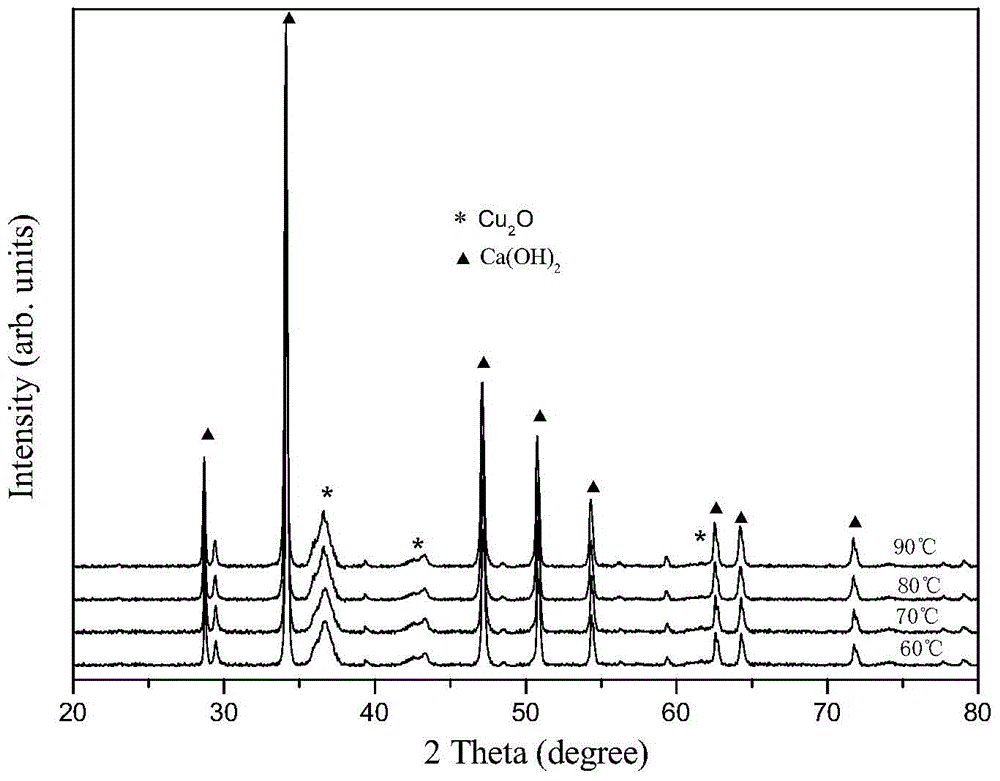
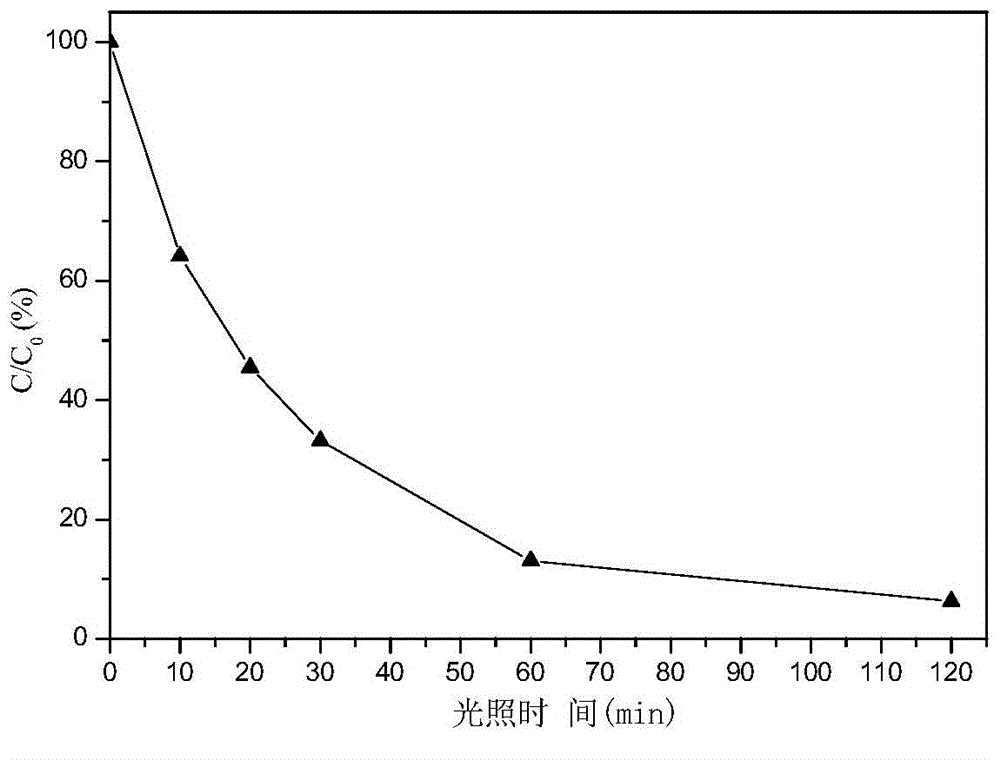
[0025] Weigh 0.01 mole of analytically pure CuCl according to the molar ratio of 0.1:1:5 2 2H 2 O, 0.1 molar analytically pure glucose and 0.5 molar analytically pure Ca(OH) 2 It was added to 27.8 mol deionized water, stirred at room temperature for 1 hour, and a suspension was obtained. The resulting suspension was stirred and heated at 80° C. for 0.5 hour, and cooled naturally to room temperature to obtain a reaction product. The resulting reaction product was suction filtered, washed with deionized water, and vacuum-dried at 60°C and 0.1MPa vacuum for 2 hours to obtain Cu 2 O / Ca(OH) 2 nanocomposite photocatalyst, where Cu 2 O average grain size is 19.7nm.
Embodiment 2
[0027] Weigh 0.03 moles of analytically pure CuCl according to the molar ratio of 0.3:1:5 2 2H 2 O, 0.1 molar analytically pure glucose and 0.5 molar analytically pure Ca(OH) 2 Added to 27.8 M deionized water, stirred at room temperature for 1 hour to obtain a suspension. The resulting suspension was stirred and heated at 80° C. for 0.5 hour, and cooled naturally to room temperature to obtain a reaction product. The resulting reaction product was suction filtered, washed with deionized water, and vacuum-dried at 60°C and 0.1MPa vacuum for 2 hours to obtain Cu 2 O / Ca(OH) 2nanocomposite photocatalyst, where Cu 2 O average grain size is 17.5nm.
Embodiment 3
[0029] Weigh 0.05 moles of analytically pure CuCl according to the molar ratio of 0.5:1:5 2 2H 2 O, 0.1 molar analytically pure glucose and 0.5 molar analytically pure Ca(OH) 2 Added to 27.8 M deionized water, stirred at room temperature for 1 hour to obtain a suspension. The resulting suspension was stirred and heated at 80° C. for 0.5 hour, and cooled naturally to room temperature to obtain a reaction product. The resulting reaction product was suction filtered, washed with deionized water, and vacuum-dried at 60°C and 0.1MPa vacuum for 2 hours to obtain Cu 2 O / Ca(OH) 2 nanocomposite photocatalyst, where Cu 2 O average grain size is 15.3nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com