Vitamin D2 and calcium levulinate injection composition and preparation method thereof
A technology of calcium fructonate and vitamins, which is applied in the field of medicine, can solve the problems of inability to guarantee sterile injection and high risk of use, and achieve the effects of excellent stability, increased safety, and guaranteed product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] one of the vitamin D 2 and the preparation method of calcium fructonate injection composition, the steps are as follows:
[0027] ①According to vitamin D 2 The weight ratio to calcium fructonate is 1:23-32, vitamin D 2 The weight ratio with dipalmitoylphosphatidylcholine is 1:40-80, vitamin D 2 The weight ratio with cholesterol is 1:24-40, Vitamin D 2 The weight ratio with distearoylphosphatidylcholine is 1:24-48, Vitamin D 2 1:64-120 by weight with polyethylene glycol, vitamin D 2 The weight-to-volume ratio of water for injection is 1 mg: 8-12 ml, and the material is taken for later use;
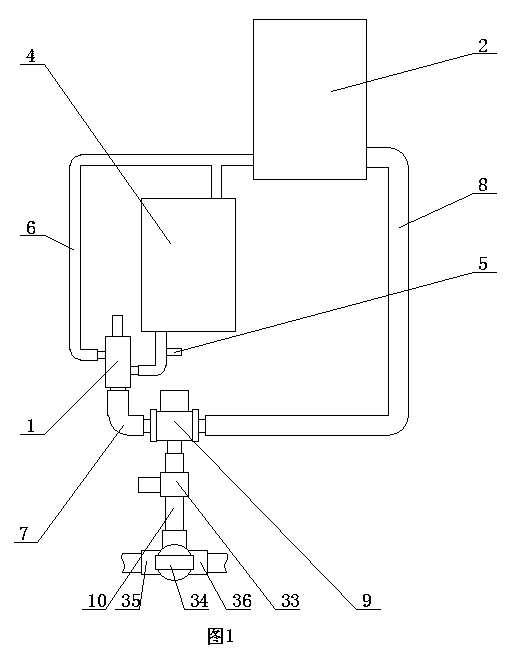
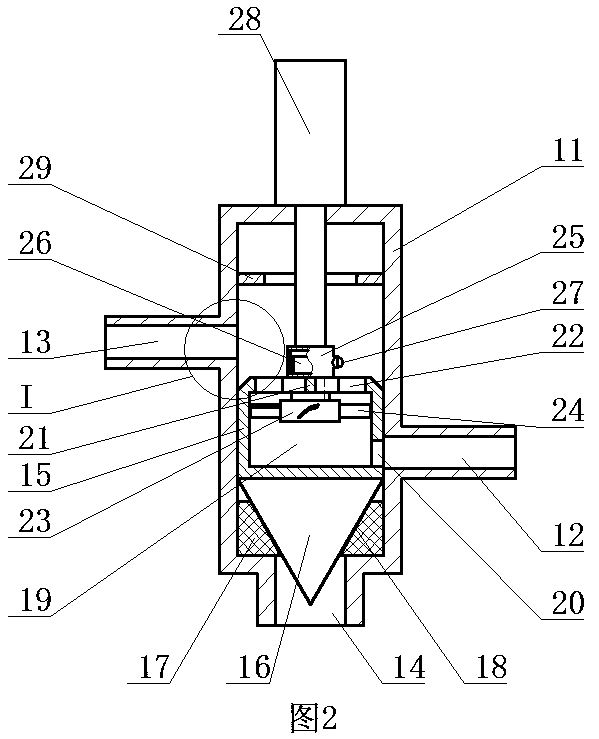
[0028] ②Connect the first water outlet of the three-way reversing valve in the constant temperature water supply system to the reactor, and the second water outlet of the three-way reversing valve to the homogenizer;
[0029] ③ Take calcium fructonate according to the ratio of step ① and put it into water for injection to dissolve. The amount of water for injection is 10% of th...
Embodiment 1
[0040] Embodiment 1, vitamin D 2 And the composition of calcium fructonate injection composition is:
[0041] Vitamin D 2 0.125g, 3.82g of calcium fructonate, 8g of dipalmitoylphosphatidylcholine, 5g of distearoylphosphatidylcholine, 3g of cholesterol, 12g of polyethylene glycol, pH value 7, dilute to 1000ml with water for injection.
Embodiment 2
[0042] Embodiment 2, vitamin D 2 And the composition of calcium fructonate injection composition is:
[0043] Vitamin D 2 0.125g, calcium fructonate 2.87g, dipalmitoylphosphatidylcholine 5g, distearoylphosphatidylcholine 6g, cholesterol 4g, polyethylene glycol 12g, pH value 7.1, dilute to 1000ml with water for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com