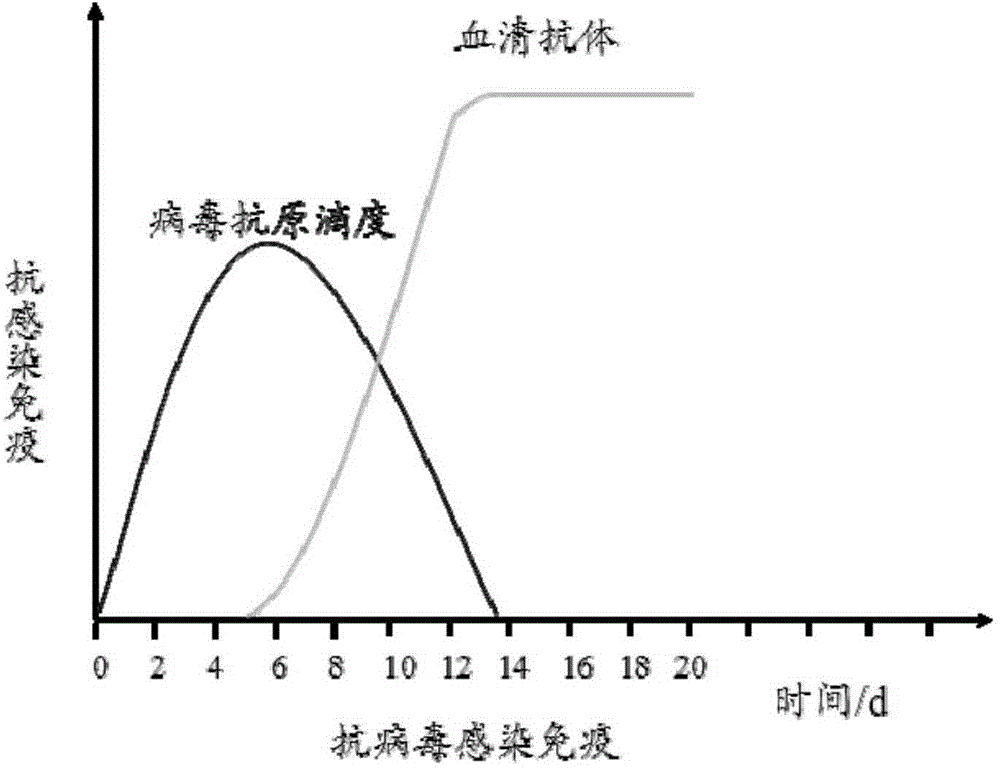
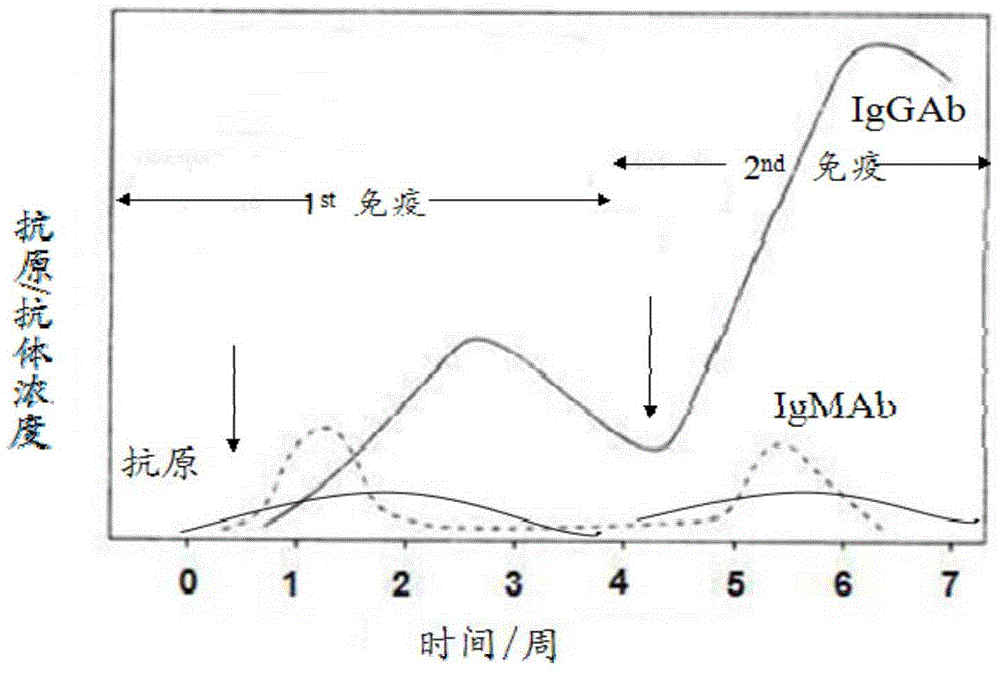
Colloidal gold immunochromatography test strip for simultaneously detecting viral antigens and antibodies
An immunochromatographic test strip and virus antigen technology, which is applied in measurement devices, analytical materials, instruments, etc., can solve the problem of not detecting influenza virus antigens at the same time, and achieve fast and intuitive reading results, low price and strong specificity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Influenza A virus detection
[0048] Detection of Influenza A Virus Antigen:
[0049] (1) Take 200 μg of influenza A labeled antibody and place it in a dialysis bag for dialysis against 5mM Tris-HCl for 24 hours. During this process, change the water every 2 hours. Ultrapure water to 2mL, discard precipitated impurities after centrifugation.
[0050] (2) Preparation of gold-labeled antibody conjugate pads: colloidal gold was prepared by reducing chloroauric acid with sodium citrate, and the glass instruments used were pre-soaked with washing solution overnight, and then rinsed with deionized water. Add 1000mL of ultrapure water to the beaker, then add 10mL of 1% sodium citrate solution, heat to boiling, then add 10mL of 1% chloroauric acid solution, boil for 15min, cool down, and store at 4°C. The particle diameter is about 20-30nm (such as Figure 14 ), put 20mL colloidal gold solution in a beaker, add 200μL 0.01M K 2 CO 3 Solution Adjust the pH of t...
Embodiment 2
[0059] Embodiment 2: Influenza B virus detection
[0060] Detection of influenza B virus antigen:
[0061] (1) Take 200 μg of influenza B labeled antibody in a dialysis bag and dialyze against 5mM Tris-HCl for 24 hours. During this process, change the water every 2 hours. After the dialysis is completed, take out the antibody and place it in a centrifuge tube, add Ultrapure water to 2mL, discard precipitated impurities after centrifugation.
[0062] (2) Preparation of gold-labeled antibody conjugate pads: colloidal gold was prepared by reducing chloroauric acid with sodium citrate, and the glass instruments used were pre-soaked with washing solution overnight, and then rinsed with deionized water. Add 1000mL of ultrapure water to the beaker, then add 10mL of 1% sodium citrate solution, heat to boiling, then add 10mL of 1% chloroauric acid solution, boil for 15min, cool down, and store at 4°C. The particle diameter is about 20-30nm, take 20mL colloidal gold solution and put i...
Embodiment 3
[0070] Embodiment 3: detection of adenovirus
[0071] Detection of Adenovirus Antigen:
[0072] (1) Take 200 μg of labeled antibody against adenovirus and place it in a dialysis bag for dialysis against 5mM Tris-HCl for 24 hours. During this process, change the water every 2 hours. After the dialysis is completed, take out the antibody and place it in a centrifuge tube. Ultrapure water to 2mL, discard precipitated impurities after centrifugation.
[0073] (2) Preparation of gold-labeled antibody conjugate pads: colloidal gold was prepared by reducing chloroauric acid with sodium citrate, and the glass instruments used were pre-soaked with washing solution overnight, and then rinsed with deionized water. Add 1000mL of ultrapure water to the beaker, then add 10mL of 1% sodium citrate solution, heat to boiling, then add 10mL of 1% chloroauric acid solution, boil for 15min, cool down, and store at 4°C. The particle diameter is about 20-30nm, take 20mL colloidal gold solution and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com