The preparation method of 1-amino-3,5-dinitropyrazole
A technology of dinitropyrazole and dinitropyrazole sodium, which is applied in directions such as organic chemistry, can solve the problems of complicated synthesis method and long synthesis time, and achieves simple preparation method, short reaction time, and simple reaction process operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
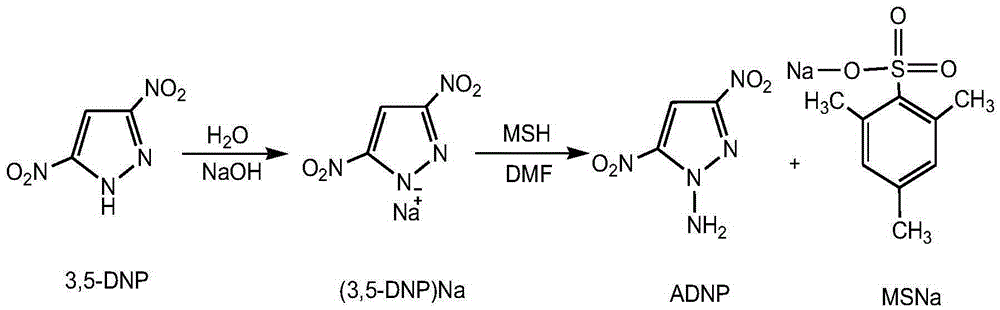
[0027] (1) Preparation of intermediate 3,5-dinitropyrazole sodium salt
[0028] Dissolve 3,5-dinitropyrazole (1.58g, 0.01mol) and NaOH (0.40g, 0.01mol) in 40mL of distilled water, stir at room temperature for 1.0h, and spin dry under vacuum to obtain the intermediate 3,5-di Nitropyrazole sodium salt [(3,5-ADNP)Na], yield 97.5%.
[0029] (2) Preparation of 1-amino-3,5-dinitropyrazole (ADNP)
[0030] Under the condition of ice bath, the intermediate (3,5-ADNP)Na (1.44g, 0.008mol) was dissolved in 40mL of anhydrous DMF, stirred, and the pre-prepared MSH (4.3g, 0.02mol) was added dropwise into anhydrous Aqueous DMF (25 mL) solution. Then rise to room temperature and react for 5.0h. In the reaction solution after the reaction, there are target product 1-amino-3,5-dinitropyrazole (ADNP) and by-product sodium trimethylbenzenesulfonate (MSNa). After ester washing, precipitates were precipitated (precipitated as the by-product sodium trimethylbenzenesulfonate), filtered, and the fi...
Embodiment 2
[0035] (1) Preparation of intermediate 3,5-dinitropyrazole sodium salt
[0036] Dissolve 3,5-dinitropyrazole (12.64g, 0.08mol) and NaOH (3.20g, 0.08mol) in 320mL of distilled water, stir at room temperature for 1.0h, and spin dry under vacuum to obtain the intermediate 3,5-di Nitropyrazole sodium salt [(3,5-ADNP)Na], yield 98.0%.
[0037] (2) Preparation of 1-amino-3,5-dinitropyrazole (ADNP)
[0038]Under the condition of ice bath, the intermediate (3,5-ADNP)Na (11.52g, 0.064mol) was dissolved in 160mL of anhydrous DMF, stirred, and the pre-prepared MSH (34.4g, 0.16mol) was added dropwise into anhydrous Aqueous DMF (200 mL) solution. Then rise to room temperature and react for 5.0h. Distilled under reduced pressure, washed with 1000 mL of ethyl acetate, precipitated out, filtered, collected the filtrate, and distilled under reduced pressure to obtain 8.42 g of ADNP crude product in the form of light yellow powder, with a yield of 60.8% and a melting point of 111-113°C.
[...
Embodiment 3
[0042] (1) Preparation of intermediate 3,5-dinitropyrazole sodium salt
[0043] Dissolve 3,5-dinitropyrazole (63.2 g, 0.4 mol) and NaOH (16.0 g, 0.4 mol) in 1500 mL of distilled water, stir at room temperature for 1.0 h, and spin dry under vacuum to obtain the intermediate 3,5-di Nitropyrazole sodium salt [(3,5-ADNP)Na], yield 96.0%.
[0044] (2) Preparation of 1-amino-3,5-dinitropyrazole (ADNP)
[0045] Under the condition of ice bath, the intermediate (3,5-ADNP)Na (57.6g, 0.32mol) was dissolved in 1500mL of anhydrous DMF, stirred, and the pre-prepared MSH (172.0g, 0.8mol) was added dropwise into anhydrous Aqueous DMF (1000 mL) solution. Then rise to room temperature and react for 5.0h. Distilled under reduced pressure, washed with 1500 mL ethyl acetate, precipitated out, filtered, collected the filtrate, and distilled under reduced pressure to obtain 43.0 g of ADNP crude product in the form of light yellow powder, with a yield of 62% and a melting point of 111-113°C.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com