Drug-carrying liposome co-modified by folic acid and TAT peptide and preparation method thereof
A liposome and co-modification technology, which is applied in the field of drug-loaded liposomes co-modified with folic acid and TAT peptide and its preparation field, can solve the problems of limited application and lack of cell selectivity of cell-penetrating peptides, and improve the transport efficiency. , the density is easy to control, the effect of reducing steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] (1) Weigh 0.8g of phospholipid soybean lecithin and 0.2g of cholesterol, dissolve it in an organic solvent of chloroform:ethanol=2:1 (volume ratio), spin dry the organic solvent under reduced pressure at 40°C, and obtain a drug-free lipid film ;
[0051] (2) Add pH 7.6 phosphate buffer solution to the drug-free lipid membrane to dissolve, sonicate for 2 minutes, pressurize the 0.22 μm membrane 10 times to obtain blank liposomes, and further adjust the pH to 7.6 with disodium hydrogen phosphate aqueous solution , add 0.15 g of doxorubicin, mix at 70°C for 30 min, and cool to room temperature to prepare drug-loaded liposomes;
[0052] (3) Subsequently, weigh DSPE-PEG 5000 -FA 10g and DSPE-PEG 2000 -TAT 5g, dissolved in phosphate buffer solution, dropwise added to the drug-loaded liposome in step (2), kept at 55°C for 1 hour, and cooled to prepare a dual-target drug-loaded liposome.
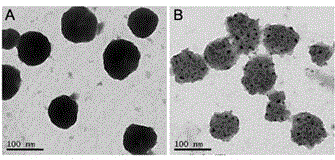
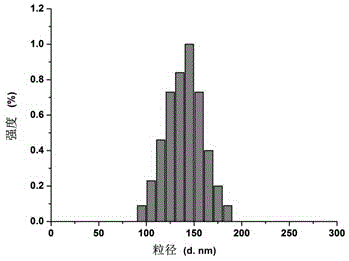
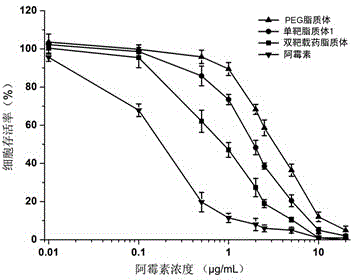
[0053] The double-target drug-loaded liposome obtained in Example 1 is tested, and...
Embodiment 2
[0055] (1) Weigh 0.8g hydrogenated soybean lecithin, 0.2g cholesterol, DSPE-PEG 3400 -FA 1g, DSPE-PEG 3000 - 1g of TAT and 0.2g of hydroxycamptothecin were dissolved in an organic solvent of chloroform:ethanol=2:1 (volume ratio), and then the organic solvent was spin-dried under reduced pressure at 40°C to obtain a drug-containing lipid film;
[0056] (2) Add pH 7.6 phosphate buffer solution to the drug-containing lipid film to dissolve, sonicate for 2 minutes, and filter 10 times with a 0.22 μm membrane to obtain dual-target drug-loaded liposomes.
Embodiment 3
[0058] (1) Weigh 0.8g of lecithin, 0.2g of cholesterol and 0.01g of paclitaxel, dissolve them in an organic solvent of chloroform:ethanol=2:1 (volume ratio), spin dry the organic solvent under reduced pressure at 40°C, and obtain the drug-containing Lipid film;
[0059] (2) Add pH 7.6 phosphate buffer solution to the drug-containing lipid film to dissolve, sonicate for 2 minutes, and filter 10 times with a 0.22 μm membrane to obtain drug-loaded liposomes;
[0060] (3) Weigh DSPE-PEG 1000 -FA 10g and DSPE-PEG 750 -TAT5g, dissolved in phosphate buffer solution, dropwise dropped into the drug-loaded liposome in step (2), kept warm, cooled, to obtain dual-target drug-loaded liposome.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com