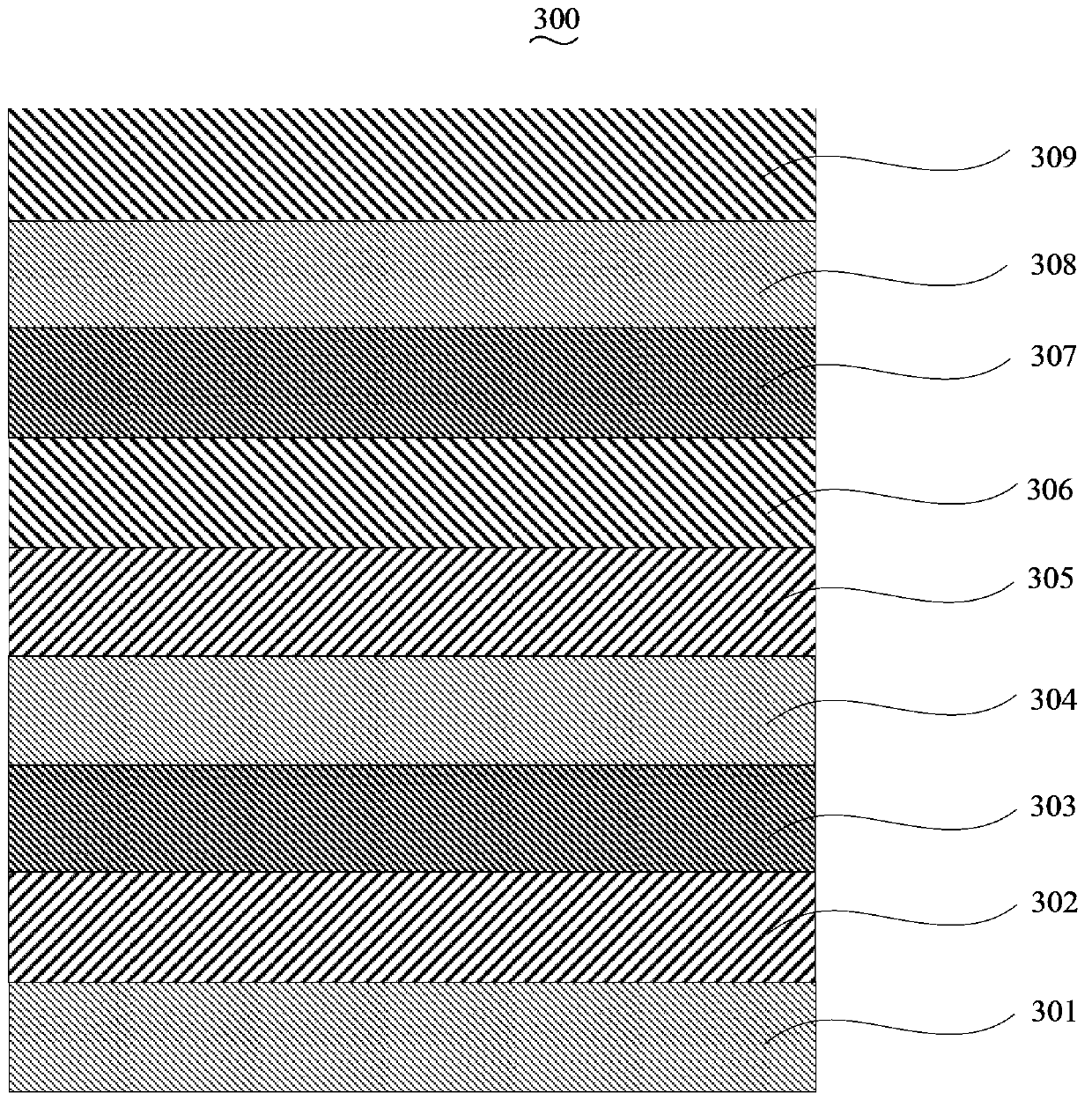
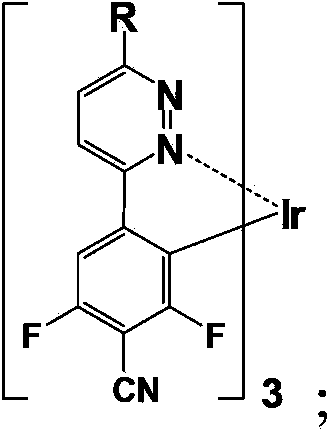
Blue light-emitting organic electroluminescent material, its preparation method and organic electroluminescent device
A luminescent and electromechanical technology, applied in luminescent materials, electrical solid devices, organic chemistry, etc., can solve problems such as poor blue light color purity, luminous color purity, luminous efficiency device efficiency attenuation bottleneck, etc., to reduce self-quenching phenomenon , Conducive to evaporation, improve the effect of luminous performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The preparation method of the above-mentioned blue-light organic electroluminescent material comprises the following steps:
[0039] S1. In an oxygen-free environment (in the present invention, the oxygen-free environment is at least one of nitrogen and argon, preferably nitrogen, the same below), the structural formula is Compound A (due to the different substituent groups of R, its chemical name is different, please refer to each embodiment for details, the same below) and the structural formula is Compound B (3,5-difluoro-4-cyanophenylboronic acid) was dissolved in the solvent, and tetrabutylammonium bromide, base and rake catalyst were added to the solvent for phase transfer, Suzuki reflux coupling React for 4 or 5h, stop the reaction, obtain the structural formula after separation and purification treatment: Compound C (due to the different substituent groups of R, its chemical name is different, see the examples for details, the same below); wherein, the molar ...
Embodiment 1
[0046] Example 1: Complex tris(3-(3',5'-difluoro-4'-cyanophenyl)pyridazine-N,C 2 ’) Synthesis of iridium
[0047] (1) Synthesis of 3-(3',5'-difluoro-4'-cyanophenyl)pyridazine
[0048]
[0049] Under nitrogen protection, 3.18g (20mmol) 3-bromopyridazine, 4.39g (24mmol) 3,5-difluoro-4-cyanophenylboronic acid, 80mL DMF, 20mL water, 3.22g (10mmol) tetrabutyl bromide Ammonium chloride (BATA), 5.53g (40mmol) anhydrous potassium carbonate, 0.23g (0.2mmol) tetrakis (triphenylphosphine) palladium (Pd (PPh 3 ) 4 ), stirred and refluxed for 4h. After the reaction solution was cooled to room temperature, it was extracted with dichloromethane, separated, washed with water until neutral, and dried over anhydrous magnesium sulfate. After filtration, the filtrate was distilled off the solvent under reduced pressure to obtain the crude product. Silica gel column chromatography was carried out with dichloromethane as the eluent. After drying, 2.95 g of solid was obtained with a yield of...
Embodiment 2
[0067] Example 2: Complex tris(3-(3',5'-difluoro-4'-cyanophenyl)-6-methylpyridazine-N,C 2 ’) Synthesis of iridium
[0068] (1) Synthesis of 3-(3',5'-difluoro-4'-cyanophenyl)-6-methylpyridazine
[0069]
[0070] Under nitrogen protection, 3.46g (20mmol) 3-bromo-6-methylpyridazine, 4.39g (24mmol) 3,5-difluoro-4-cyanophenylboronic acid, 80mL N,N-dimethylformamide , 20mL of water, 3.22g (10mmol) of tetrabutylammonium bromide, 8.48g (80mmol) of anhydrous sodium carbonate, 1.15g (1mmol) of tetrakis (triphenylphosphine) palladium, stirred and refluxed for 4h. After the reaction solution was cooled to room temperature, it was extracted with dichloromethane, separated, washed with water until neutral, and dried over anhydrous magnesium sulfate. After filtration, the filtrate was distilled off the solvent under reduced pressure to obtain the crude product. Silica gel column chromatography was carried out with dichloromethane as the eluent. After drying, 3.00 g of solid was obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com