Reaction system for halogenating ion exchange resins and halogenation method of ion exchange resins
A technology of ion exchange resin and reaction system, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem of high toxicity, high price and easy leakage of halogens, etc. problem, to achieve the effect of reducing the sulfonic acid group shedding rate, improving thermal stability, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
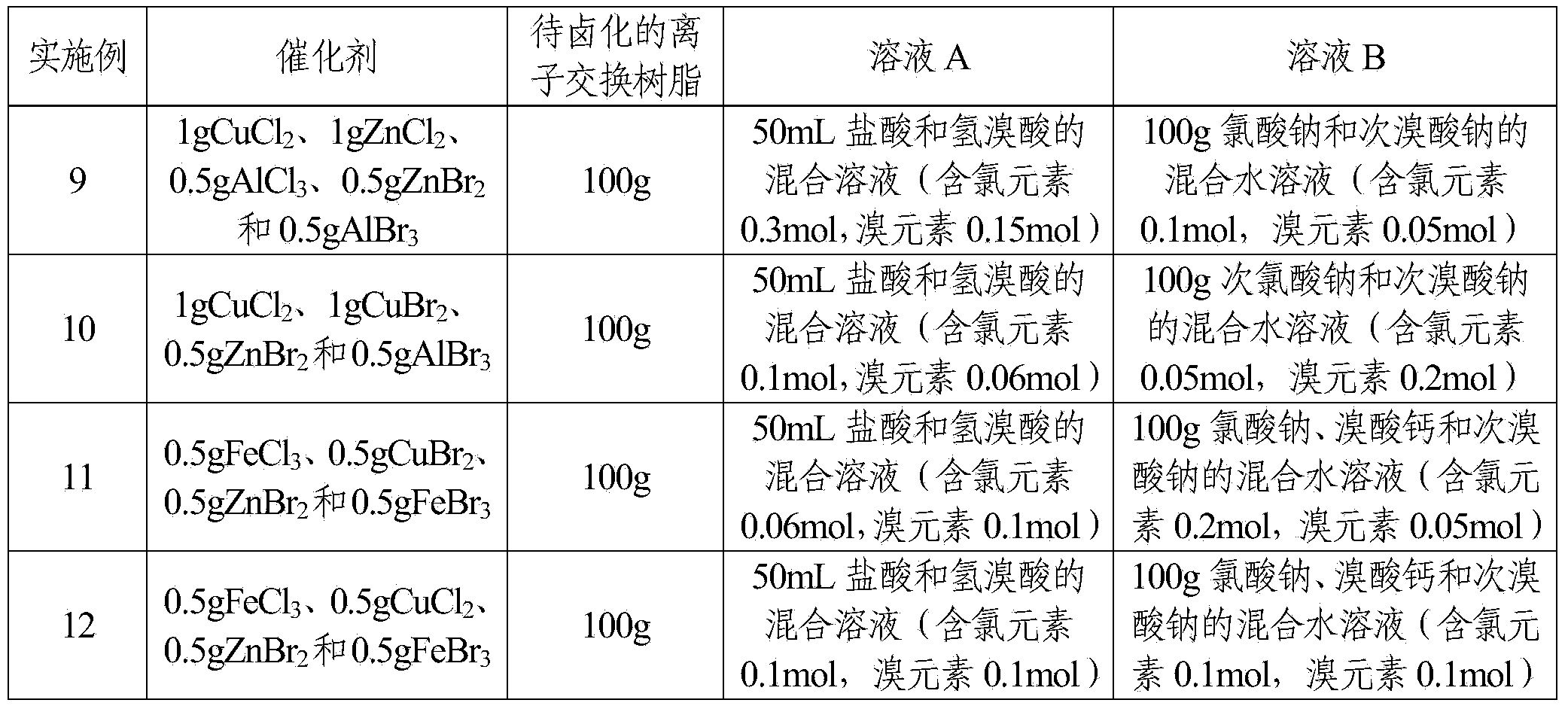
Examples
Embodiment 1
[0029] The reaction system for the halogenated ion exchange resin of the present embodiment comprises a catalyst and a halogenating reagent, and the catalyst is FeBr 3 The quality of the catalyst is 1% of the quality of the ion-exchange resin to be halogenated; the halogenation reagent includes solution A and solution B, and the solution A is the hydrobromic acid of 6mol / L, and the solution B is a mass concentration of 25 % sodium bromate aqueous solution; the mol ratio of the bromine element in the solution A and the bromine element in the solution B is 2.4: 1; the quality of the solute in the solution B is 37.5% of the ion exchange resin mass to be halogenated.
[0030] The halogenation method of the present embodiment is:
[0031] Step 1. Swell 100g of styrene-based resin white balls with 150mL of dichloroethane at room temperature for 2 hours, filter and bake at 60°C until the surface is dry to obtain a swelled ion exchange resin;
[0032] Step 2, place the ion exchange r...
Embodiment 2
[0036] The reaction system for the halogenated ion exchange resin of the present embodiment includes a catalyst and a halogenation reagent, and the catalyst is CuCl 2 The quality of the catalyst is 0.1% of the mass of the ion-exchange resin to be halogenated; the halogenation reagent includes solution A and solution B, and the solution A is 2mol / L hydrochloric acid, and the solution B is 30% of the mass concentration Potassium chlorate aqueous solution; the molar ratio of the chlorine element in the solution A to the chlorine element in the solution B is 2.7:1; the mass of the solute in the solution B is 18% of the mass of the ion exchange resin to be halogenated.
[0037] The halogenation method of the present embodiment is:
[0038] Step 1. Swell 100g of styrene-based resin white balls with 200mL of carbon tetrachloride at 50°C for 0.5h, filter and bake at 70°C until the surface is dry to obtain a swelled ion exchange resin;
[0039] Step 2, place the ion exchange resin of ...
Embodiment 3
[0043] The reaction system for the halogenated ion exchange resin of the present embodiment includes a catalyst and a halogenating reagent, and the catalyst is CuBr with a mass ratio of 1:1. 2 and FeBr 3The quality of the catalyst is 5% of the ion-exchange resin quality to be halogenated; The halogenation reagent includes solution A and solution B, and the solution A is the hydrobromic acid of 12mol / L, and the solution B is potassium bromate and high The mixed aqueous solution of sodium bromate, wherein the mass concentration of potassium bromate is 10%, and the mass concentration of sodium perbromate is 20%; The mol ratio of the bromine element in the described solution A and the bromine element in the solution B is 3: 1; The mass of the solute in the solution B is 30% of the mass of the ion exchange resin to be halogenated.
[0044] The halogenation method of the present embodiment is:
[0045] Step 1. Swell 100g of styrene-based resin white balls with 180mL of dichloroeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com