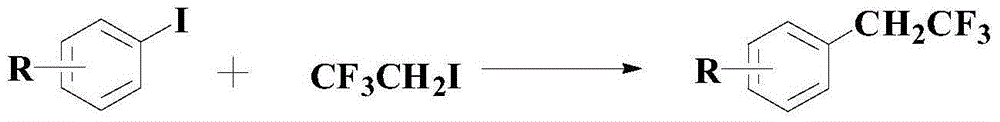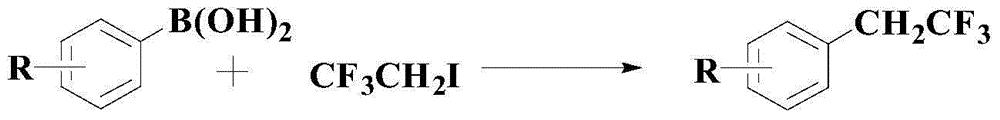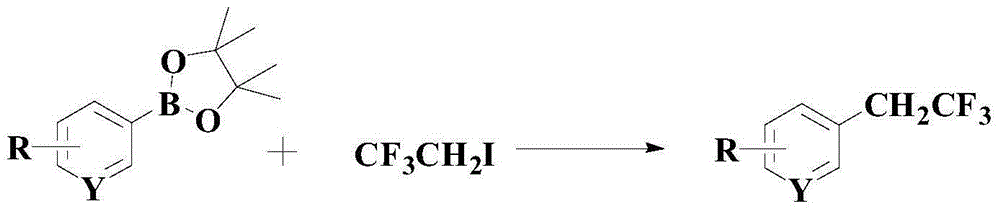A kind of synthetic method of the medicine intermediate compound containing trifluoroethyl
A synthetic method, trifluoroethyl technology, applied in the synthesis of pharmaceutical intermediate compounds, the field of synthesis of pharmaceutical intermediates, can solve the problems of harsh reaction conditions, low reaction yield, etc., to improve the reaction yield, overcome the yield low rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] Under the atmosphere of argon gas, add 1mmol formula (I) compound, 2.2mmol 120 mesh copper powder and 4.5ml solvent DMF to the reactor, then add 3mmol CF 3 CH 2 1, finally adding 80mg mass ratio is the auxiliary agent mixture of tricyclohexylphosphine, zirconium dioxide and ethylene glycol di-n-octyl sodium succinate sulfosuccinate of 1:0.4:1.3, keep the inert atmosphere after continuing to evacuate for 10min and The temperature was raised to 95°C and the reaction was stirred for 18 hours. After the reaction was completed, it was cooled and ethyl acetate was added to the mixture. After filtration, the product layers were combined, washed with water, and dried with anhydrous magnesium sulfate. The organic layer was concentrated in vacuo, and the residue was purified by silica gel column chromatography. The compound of formula (II) can be obtained with a yield of 98.5% and a purity of 99.3% (HPLC).
[0036] 1 HNMR (500MHz, CDCl 3 )δ7.35(d, J=8.3Hz, 2H), 7.2...
Embodiment 2
[0038]
[0039] Under argon gas atmosphere, add 1mmol formula (I) compound, 2.5mmol 100 mesh copper powder and 4ml solvent DMF to the reaction kettle, then add 3.3mmol CF 3 CH 2 1, finally adding 90mg mass ratio is the auxiliary agent mixture of tricyclohexylphosphine, zirconium dioxide and ethylene glycol di-n-octyl sodium succinate sulfosuccinate of 1:0.4:1.3, keep the inert atmosphere after continuing to evacuate for 10min and The temperature was raised to 100°C and the reaction was stirred for 24 hours. After the reaction was completed, it was cooled and ethyl acetate was added to the mixture. After filtration, the product layers were combined, washed with water, and dried with anhydrous magnesium sulfate. The organic layer was concentrated in vacuo, and the residue was purified by silica gel column chromatography. The compound of formula (II) can be obtained with a yield of 98.9% and a purity of 99.1% (HPLC).
[0040] 1 HNMR (400MHz, CDCl 3 )δ7.65(d, J=8.3Hz, 2H), 7.4...
Embodiment 3
[0042]
[0043] Under argon gas atmosphere, add 1mmol formula (I) compound, 2.4mmol 150 mesh copper powder and 5ml solvent DMF to the reaction kettle, then add 3.5mmol CF 3 CH 2 1, finally adding 70mg mass ratio is the auxiliary agent mixture of tricyclohexylphosphine, zirconium dioxide and ethylene glycol di-n-octyl sodium succinate sulfosuccinate of 1:0.4:1.3, keep the inert atmosphere after continuing to evacuate for 10min and The temperature was raised to 90°C and the reaction was stirred for 22 hours. After the reaction was completed, it was cooled and ethyl acetate was added to the mixture. After filtration, the product layers were combined, washed with water, and dried with anhydrous magnesium sulfate. The organic layer was concentrated in vacuo, and the residue was purified by silica gel column chromatography. The compound of formula (II) can be obtained with a yield of 98.7% and a purity of 98.9% (HPLC).
[0044] 1 HNMR (400MHz, CDCl 3 )δ10.01(s,1H),7.86(d,J=7.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com