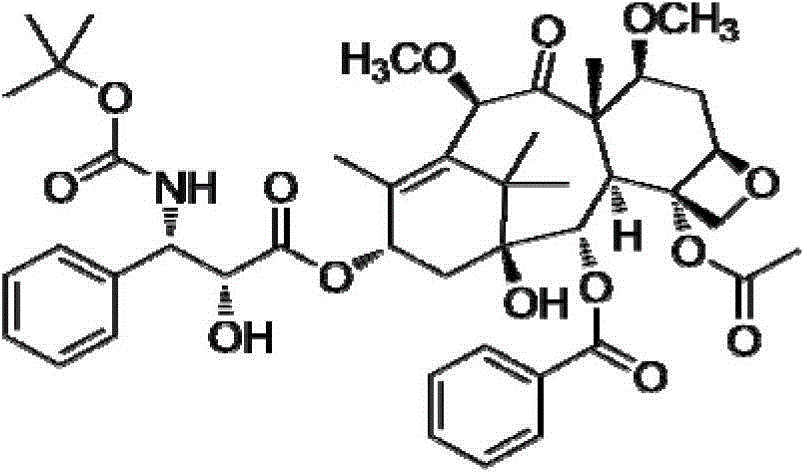
A kind of injection composition containing cabazitaxel and its preparation method
A cabazitaxel and composition technology, which is applied in the field of injection compositions, can solve problems such as large irritation, reduce ethanol, etc., and achieve the effects of simple production process, reduced solvent residual amount and related substance content, and easy industrialized operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
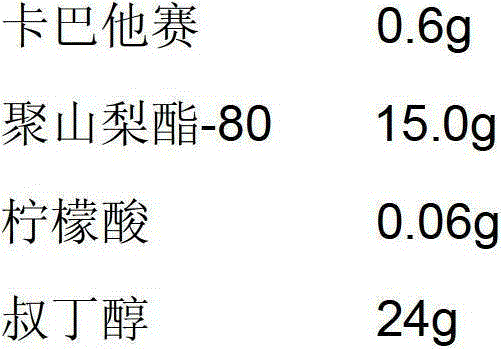
[0037]
[0038] Add the prescribed amount of polysorbate-80 and citric acid to tert-butanol and stir to dissolve, then add the prescribed amount of cabazitaxel, stir until the solution is clear, take the intermediate to test the pH and content, pass through a 0.22μm micropore The filter membrane was sterilized and filtered, and the filtrate was evenly distributed in 10 10ml vials, and half-tightened to a freeze dryer. Control the temperature of the front box of the freeze dryer at 20°C to 25°C, and the temperature of the cold trap at -80°C; turn on the vacuum pump, control the pressure of the front box at 130Pa to 150Pa, and maintain it for 4 hours; adjust the pressure of the front box at 10Pa to 20Pa, and keep it for 4 hours; do not set The front box pressure value, vacuumize, when the pressure is lower than 5Pa, close the septum valve, after the front box pressure has no significant change, fully press the plug, take out the box, tie the cover, and get the finished product...
Embodiment 2
[0040]
[0041] Add the prescribed amount of polysorbate-80 and citric acid to tert-butanol and stir to dissolve, then add the prescribed amount of cabazitaxel, stir until the solution is clear, take the intermediate to test the pH and content, pass through a 0.22μm micropore The filter membrane was sterilized and filtered, and the filtrate was evenly distributed in 10 10ml vials, and half-tightened to a freeze dryer. Control the temperature of the front box of the freeze dryer at 25°C to 30°C, and the temperature of the cold trap at -80°C; turn on the vacuum pump, control the pressure of the front box at 50Pa to 80Pa, and maintain it for 4 hours; adjust the pressure of the front box at 30Pa to 50Pa, and keep it for 3 hours; do not set The front box pressure value, vacuumize, when the pressure is lower than 5Pa, close the septum valve, after the front box pressure has no significant change, fully press the plug, take out the box, tie the cover, and get the finished product. ...
Embodiment 3
[0043]
[0044] Add the prescribed amount of polysorbate-80 and citric acid to tert-butanol and stir to dissolve, then add the prescribed amount of cabazitaxel, stir until the solution is clear, take the intermediate to test the pH and content, pass through a 0.22μm micropore The filter membrane was sterilized and filtered, and the filtrate was evenly distributed in 10 10ml vials, and half-tightened to a freeze dryer. Control the temperature of the front box of the freeze dryer at 30°C to 35°C, and the temperature of the cold trap at -80°C; turn on the vacuum pump, control the pressure of the front box at 100Pa to 120Pa, and maintain it for 3 hours; adjust the pressure of the front box at 10Pa to 20Pa, and keep it for 3 hours; do not set The front box pressure value, vacuumize, when the pressure is lower than 5Pa, close the septum valve, after the front box pressure has no significant change, fully press the plug, take out the box, tie the cover, and get the finished product...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com