Polypeptide, targeted drug carrier, preparation method of targeted drug carrier, pharmaceutical composition, and preparation method of pharmaceutical composition
A drug and carrier technology, which is applied in the direction of drug combination, pharmaceutical formula, and non-active ingredients of polymer compounds, etc., can solve the problems of low tumor targeting efficiency and low penetration rate of tumor sites, etc., and achieve high-efficiency targeting and strong tumor penetration effect of ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] In a fourth aspect, the present invention provides a method for preparing a pharmaceutical composition, wherein the method comprises:
[0034] (1) In an organic solvent, the hydrophobic drug is mixed with the substance containing the targeted drug carrier provided by the present invention, and then dried to remove the organic solvent to obtain a dried material;
[0035] (2) Contacting the dried material with a buffer solution.
[0036] According to the present invention, in step (1), the content of the targeted drug carrier in the substance containing the targeted drug carrier provided by the present invention can vary widely, for example, it can be 20-100% by weight. Wherein, the substances in the substances other than the targeted drug carrier of the present invention can be, for example, common drug carriers, that is, drug carriers not connected with maleamide groups, for example, distearoylphosphatidylethanolamine-polyethylene Diol 2000 (DSPE-PEG 2000 ).
[0037]...
Embodiment 1
[0050] This example is used to illustrate the polypeptide, targeted drug carrier, pharmaceutical composition and preparation method thereof provided by the present invention
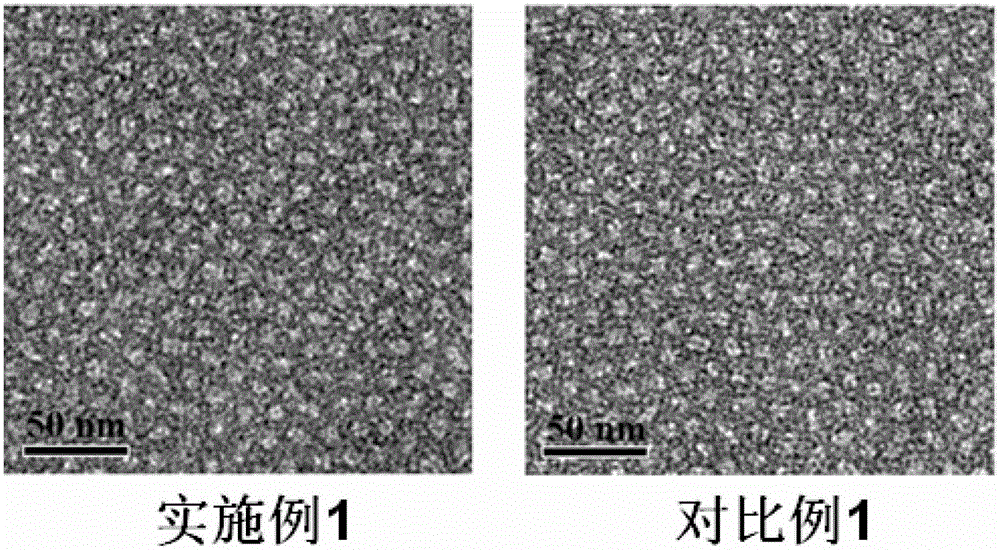
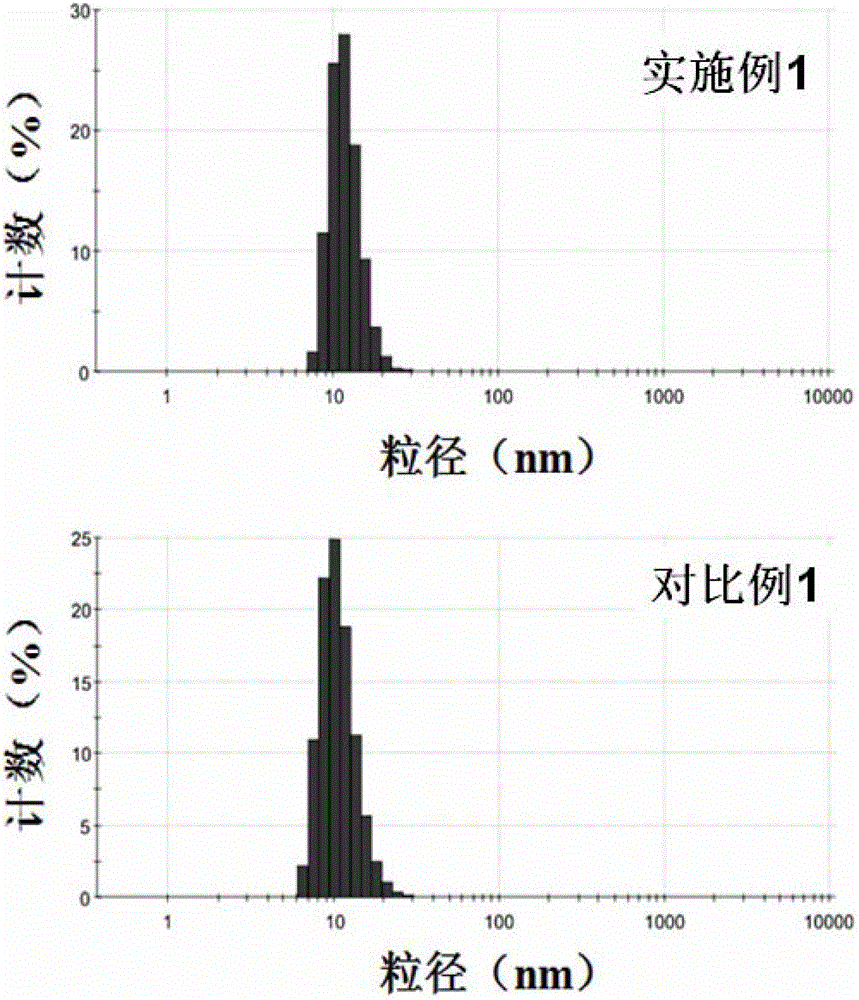
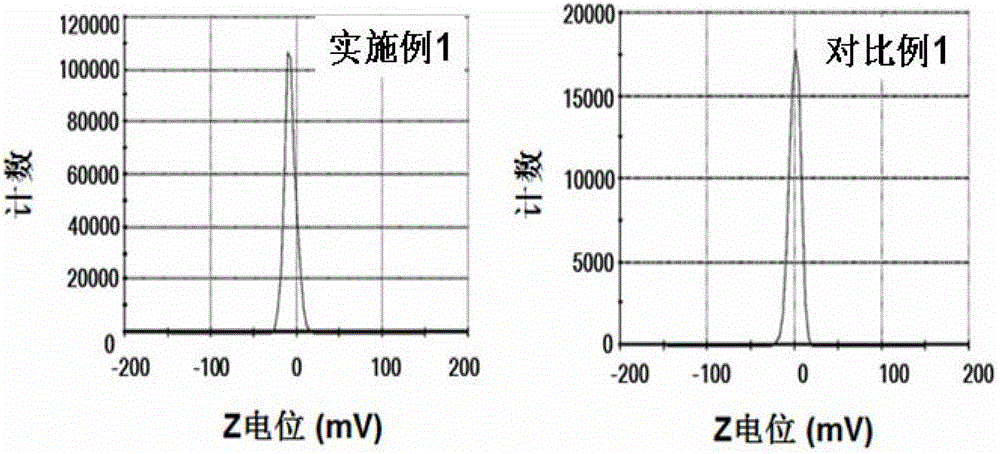
[0051] (1) Weigh 3 mg of peptide and 15 mg of DSPE-PEG respectively 2000 -MAL (the molar ratio of the polypeptide to the maleamide group in the drug carrier is 1:1) and dissolved in 2 mL of 50 mM hydroxyethylpiperazineethanesulfonic acid (HEPES) buffer (pH 6.5 ) at 30°C for 48 hours, the solution after the contact was dialyzed in a dialysis bag with a molecular weight of 2000 to remove unlinked polypeptides, and after the dialysis was completed, it was freeze-dried to obtain the drug containing the targeting drug carrier of the present invention dry powder.
[0052] (2) Put 2mg of the dry powder containing the targeted drug carrier prepared in step (1) and 8mg of the common drug carrier DSPE-PEG in an eggplant-shaped bottle 2000 and 2 mg of hydrophobic doxorubicin were dissolved in 4 mL of a mixed solu...
Embodiment 2
[0055] This example is used to illustrate the polypeptide, targeted drug carrier, pharmaceutical composition and preparation method thereof provided by the present invention
[0056] (1) Weigh 15 mg of peptide and 15 mg of DSPE-PEG respectively 2000 -MAL (the molar ratio of the polypeptide to the maleamide group in the drug carrier is 5:1) and dissolved in 3 mL of 40 mM hydroxyethylpiperazineethanesulfonic acid (HEPES) buffer (pH 6.0 ) at 20°C for 60 hours, the solution after the contact is dialyzed in a dialysis membrane with a molecular weight of 2000 to remove unlinked polypeptides, and after the dialysis is completed, it is freeze-dried to obtain the target drug carrier of the present invention dry powder.
[0057] (2) Dissolve 10 mg of the dry powder containing targeted drug carrier prepared in step (1) and 1 mg of docetaxel in 3 mL of a 2:1 mixed solution of chloroform and methanol in an eggplant-shaped bottle, and Referring to the method disclosed in Pharmaceutics (Pe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com