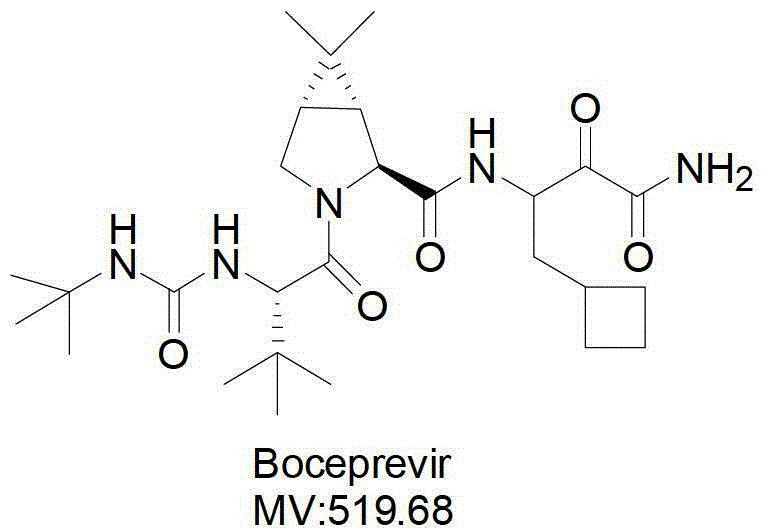
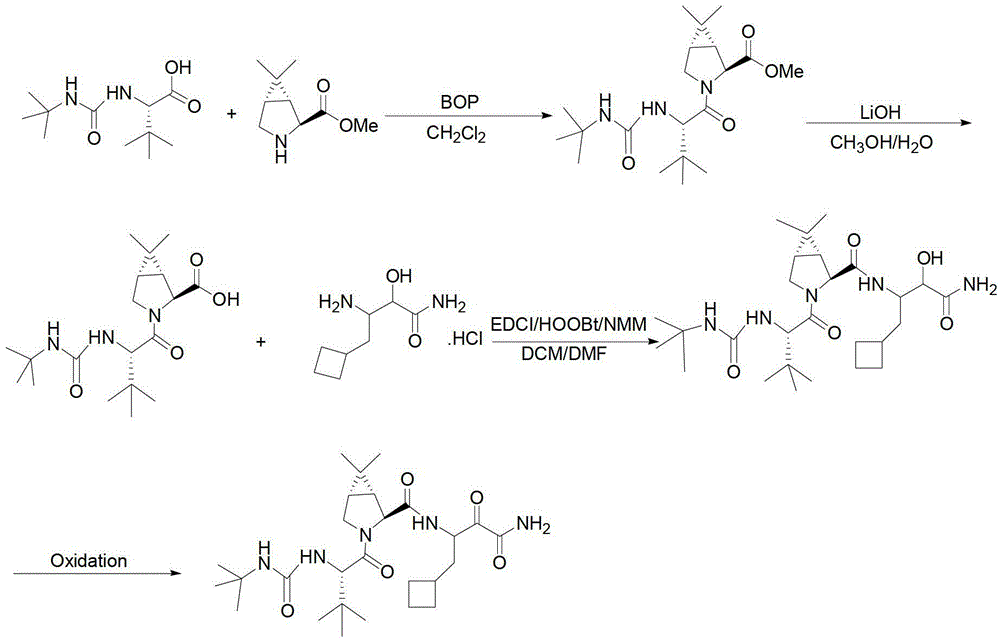
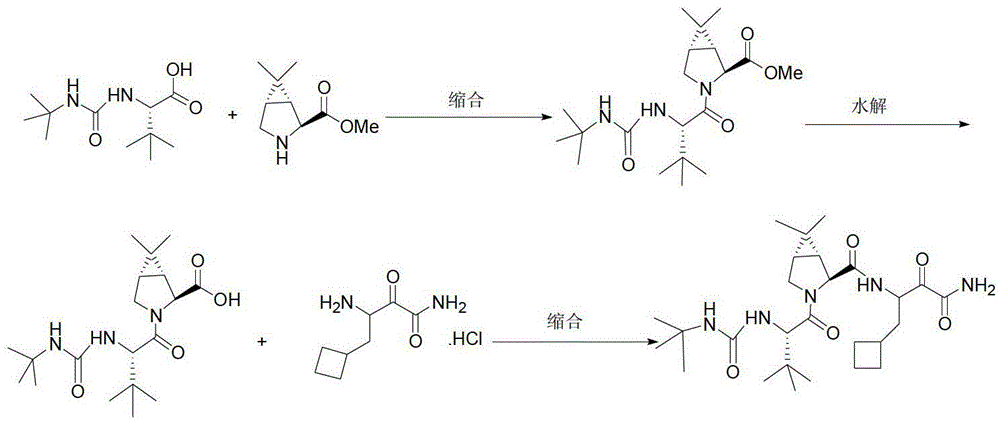
Anti-HCV drug Boceprevir intermediate II preparation method and application thereof
The technology of a compound and a compound of the general formula is applied in the field of preparation of the anti-hepatitis C drug Boceprevir, which can solve the problems of reaction detection and control of intermediates, side reactions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-9
[0068] Embodiment 1-9: R is the preparation of formula 1 compound of hydrogen
Embodiment 1
[0069] Embodiment 1: the preparation of compound B
[0070]
[0071] Compound A (5g, 20.6mmol) was dissolved in 40ml DMF and added to a three-neck flask, cooled to -5°C; NaH (60%, 1.1g, 26.8mmol) was added in batches and stirred for 1h, then added dropwise at -5°C 40ml of BnBr (4.2g, 24.7mmo) DMF solution, after the dropwise addition, stir at this temperature for 2h, add ice water to quench the reaction, extract with ethyl acetate, a small amount of multiple times, combine the organic phases, wash with saturated brine Drying over anhydrous sodium sulfate and distilling off the solvent gave a yellow oil, and column chromatography gave 6.2 g of compound B as a white solid, yield 90.4%, m / z (MH+) 334.16, 1H NMR (400 MHz, CDCl ) δ 1.31 ( t,3H),1.41-1.57(m,4H),1.64-1.74(m,2H),1.77(s,3H),1.96-2.06(m,2H),2.25-2.29(m,1H),3.95( d, 1H), 4.19-4.25(m, 3H), 4.28(d, 1H), 4.81(d, 1H), 5.63(d, 1H), 7.30-7.35(m, 5H).
Embodiment 2
[0072] Embodiment 2: the preparation of compound C
[0073]
[0074] Compound B (5g, 15mmol), DMAP (0.37g, 3mmol) were dissolved in 60ml of THF, added (Boc) 2 After O (6.88ml, 30mmol), the temperature was raised to reflux, and after reflux for about 8 hours, it was lowered to room temperature, and 60ml of methanol and hydrazine hydrate (3g, 60mmol) were added to stir for 4 hours, and then 200ml of dichloromethane was added to dilute, and the reaction solution was sequentially diluted with 1N HCl and copper sulfate solution. , washed with saturated sodium bicarbonate solution and saturated brine, dried over anhydrous sodium sulfate, evaporated to remove the solvent to obtain a yellow oil, column chromatography obtained 4.4g of compound C as a white solid, yield 75.0%, m / z (MNa+) 414.06,1H NMR(400MHz,CDCl3)δ1.32(t,3H),1.44(s,9H),1.48-1.68(m,4H),1.78-1.87(m,2H),2.02-2.11(m,2H ),2.33-2.37(m,1H),3.98(m,1H),4.20-4.32(m,3H),4.43(d,1H),4.65(d,1H),4.81(d,1H),7.30- 7.37 (m, 5H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com