Porous hydrogen storage material and preparation method thereof
A technology of hydrogen storage materials and rare earth oxides, applied in the field of porous hydrogen storage materials and their preparation, to achieve the effects of fast hydrogen absorption, easy control, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0023] 1) Dissolve 1g of sodium borohydride (NaBH 4 ) mixed with 3mL of sodium hydroxide aqueous solution with a pH of 13 to make a sodium borohydride solution, and 0.05g of cobalt chloride (CoCl 2 ) fully dissolved in 3mL water to obtain CoCl 2 aqueous solution, the CoCl 2 The aqueous solution is added to the sodium borohydride solution, and then the hydrolysis reaction is carried out at 30°C for 2 minutes. After the hydrolysis reaction is completed, it is filtered, and the filtered residue is dried in the air (drying temperature is 30°C) for 18 hours to obtain Co-B ;2) Combine Co-B with Y 2 o 3 According to the mass ratio of 1:1, stir and mix in a mortar to obtain a mixed catalyst; 3) NaBH 4 Dissolve in NaOH aqueous solution with pH value of 12 to get NaBH 4 Alkaline aqueous solution, NaBH 4 NaBH in alkaline aqueous solution 4 The mass fraction is 9%; 4) according to NaBH 4 NaBH in alkaline aqueous solution 4 content of NaBH 4 With the ratio of 50% of the total mas...
Embodiment 1-2
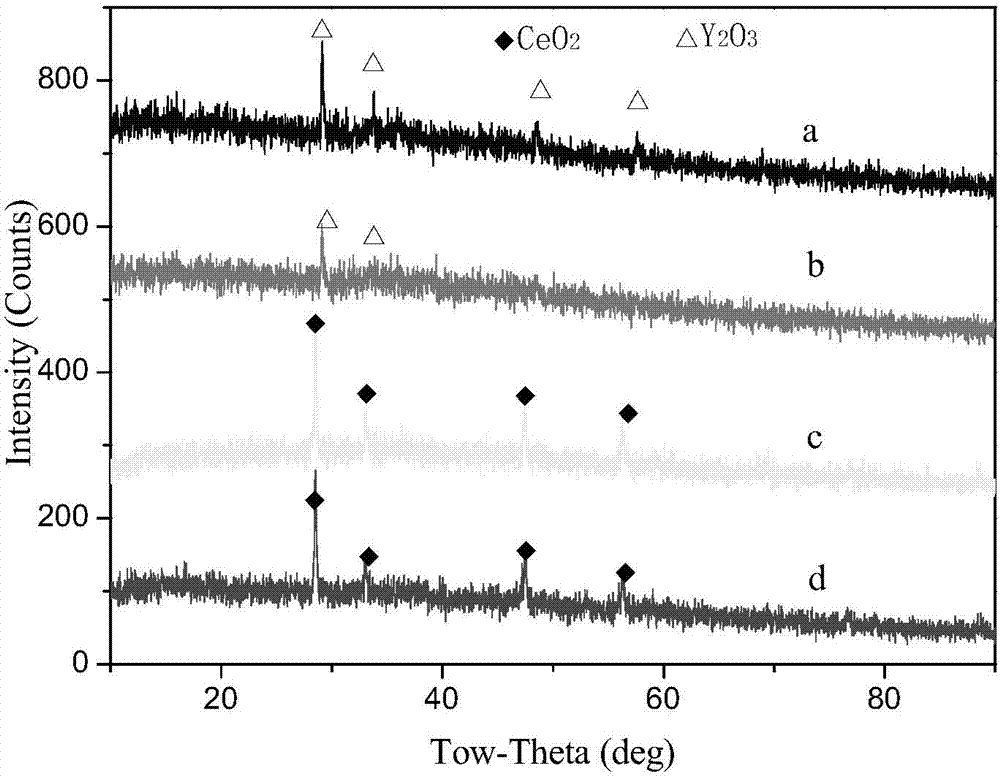
[0025] with CeO 2 replace Y 2 o 3 , others are identical with embodiment 1-1.
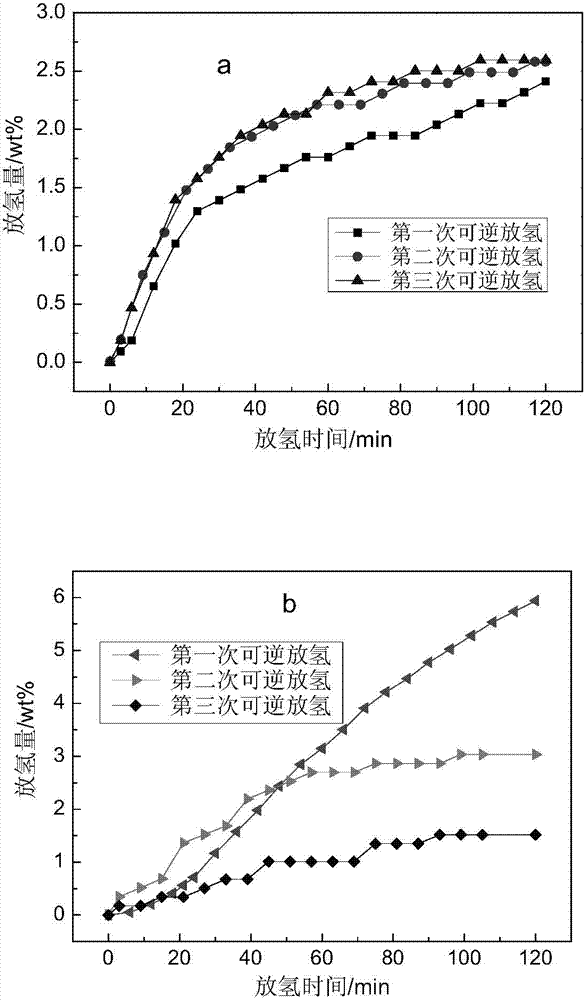
[0026] For embodiment 1-1 and 1-2, from figure 1 a It can be seen that the doped Y 2 o 3 The reversible hydrogen absorption and desorption performance of the porous hydrogen storage material is relatively stable. After three cycles, the reversible hydrogen desorption amount has no obvious attenuation. In addition, according to figure 1 a It can be seen that after the porous hydrogen storage material absorbs hydrogen for 3-5 minutes at room temperature and a hydrogen pressure of 3PMa, and then releases hydrogen in a vacuum at 150°C, the first reversible hydrogen release amount can reach 2.4wt%, indicating that the Porous hydrogen storage materials have excellent reversible hydrogen storage performance and fast hydrogen absorption under relatively mild conditions.
[0027] In addition according to figure 1 b. After hydrogen absorption at room temperature and a hydrogen pressure of 3PMa for 3-5...
Embodiment 2-1
[0031] 1) Dissolve 1.5g of sodium borohydride (NaBH 4 ) mixed with 8mL of sodium hydroxide aqueous solution with a pH of 13 to make a sodium borohydride solution, and 0.1g of cobalt chloride (CoCl 2 ) fully dissolved in 5mL water to obtain CoCl2 aqueous solution, the CoCl 2 The aqueous solution is added to the sodium borohydride solution, and then the hydrolysis reaction is carried out at 25°C for 3 minutes. After the hydrolysis reaction is completed, it is filtered, and the filtered residue is dried in the air (drying temperature is 25°C) for 12 hours to obtain Co-B ;2) Combine Co-B with Y 2 o 3 Stir and mix in a mortar at a mass ratio of 1:1.5 to obtain a mixed catalyst; 3) NaBH 4 Dissolve in NaOH aqueous solution with pH 12.5 to get NaBH 4 Alkaline aqueous solution, NaBH 4 NaBH in alkaline aqueous solution 4 The mass fraction is 9%; 4) according to NaBH 4 NaBH in alkaline aqueous solution 4 content of NaBH 4 With the ratio of 55% of the total mass of the mixed cata...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com