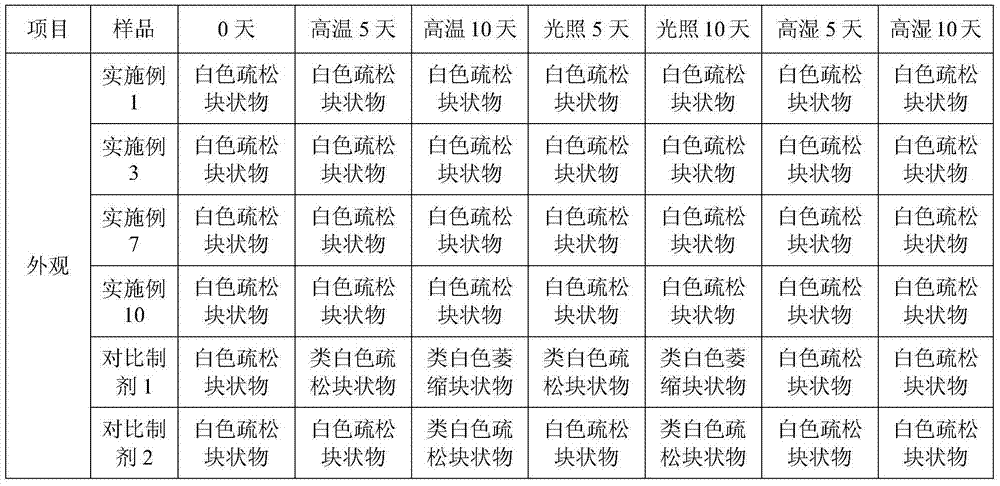
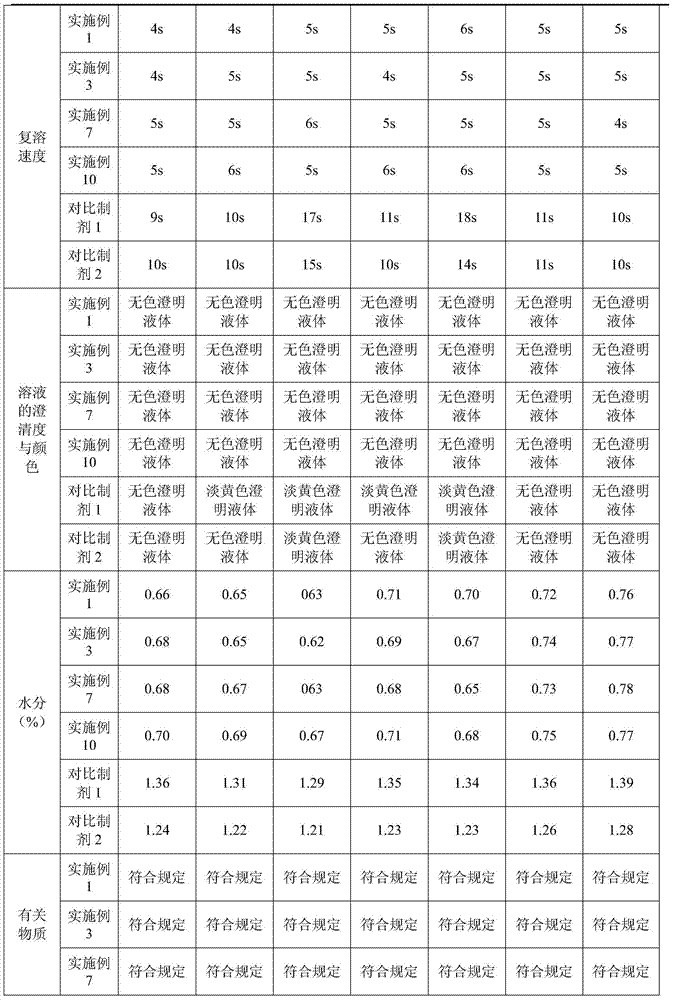
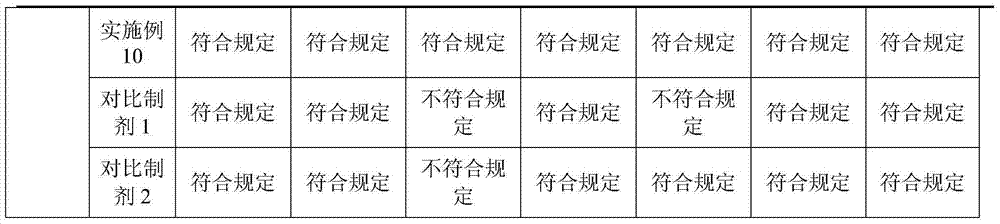
Tetracaine hydrochloride pharmaceutical composition for injection and preparation method of pharmaceutical composition
A technology of tetracaine hydrochloride and its composition, which is applied in the field of medicine, can solve problems such as the inability to judge the nitrogen gas charged, the difficulty in guaranteeing the product quality, and the inability to detect it quickly, so as to meet the needs of large-scale production, good product quality, and avoid product The effect of inconsistent quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: tetracaine hydrochloride for injection (calculated in 1000 bottles, unit: g)
[0028] tetracaine hydrochloride
[0029] Preparation process: Inject 800ml of water for injection into the liquid mixing tank, first add 0.5g of tartaric acid, stir to dissolve, then add 25g of tetracaine hydrochloride, stir to completely dissolve, add 0.1% activated carbon, keep stirring at 50°C for 15 minutes, use while hot Coarse filtration with 0.45 μm microporous membrane to obtain filtrate; add water for injection to nearly full amount, adjust pH to 4.8 with 0.1mol / L hydrochloric acid solution, add water for injection to full amount; use 0.22 μm microporous membrane for fine filtration, and conduct semi-finished product inspection, After passing the test, fill 1.0ml per bottle into 3ml vials; place in a freeze-drying box, pre-freeze at -40°C for 4 hours, start vacuuming, control the vacuum between 10-30Pa, raise the temperature to -25°C and keep for 12h , heat up...
Embodiment 2
[0030] Embodiment 2: tetracaine hydrochloride for injection (based on 1000 bottles, unit: g)
[0031] tetracaine hydrochloride
[0032] Preparation process: Inject 800ml of water for injection into the liquid mixing tank, first add 2.5g of tartaric acid, stir to dissolve, then add 25g of tetracaine hydrochloride, stir to dissolve completely, add 0.1% activated carbon, keep stirring at 50°C for 15 minutes, use while hot Coarse filtration with 0.45 μm microporous membrane to obtain filtrate; add water for injection to nearly full amount, adjust pH to 5.1 with 0.1mol / L hydrochloric acid solution, add water for injection to full amount; use 0.22 μm microporous membrane for fine filtration, and conduct semi-finished product inspection, After passing the test, fill 1.0ml per bottle into 3ml vials; place in a freeze-drying box, pre-freeze at -50°C for 1 hour, start vacuuming, control the vacuum between 10-30Pa, raise the temperature to 0°C and keep for 4 hours. Raise the te...
Embodiment 3
[0033] Embodiment 3: Prescription of tetracaine hydrochloride for injection (based on 1000 bottles, unit: g)
[0034] tetracaine hydrochloride
[0035] Preparation process: Inject 800ml of water for injection into the liquid mixing tank, first add 1.0g of tartaric acid, stir to dissolve, then add 25g of tetracaine hydrochloride, stir to completely dissolve, add 0.1% activated carbon, keep stirring at 50°C for 15 minutes, use while hot Coarse filtration with 0.45 μm microporous membrane to obtain filtrate; add water for injection to nearly full amount, adjust pH to 4.9 with 0.1mol / L hydrochloric acid solution, add water for injection to full amount; use 0.22 μm microporous membrane for fine filtration, and conduct semi-finished product inspection, After being qualified, fill 1.0ml per bottle into 3ml vials; place in a freeze-drying box, pre-freeze at -40°C for 3 hours, start vacuuming, control the vacuum between 10-30Pa, raise the temperature to -15°C and keep for 6h ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com