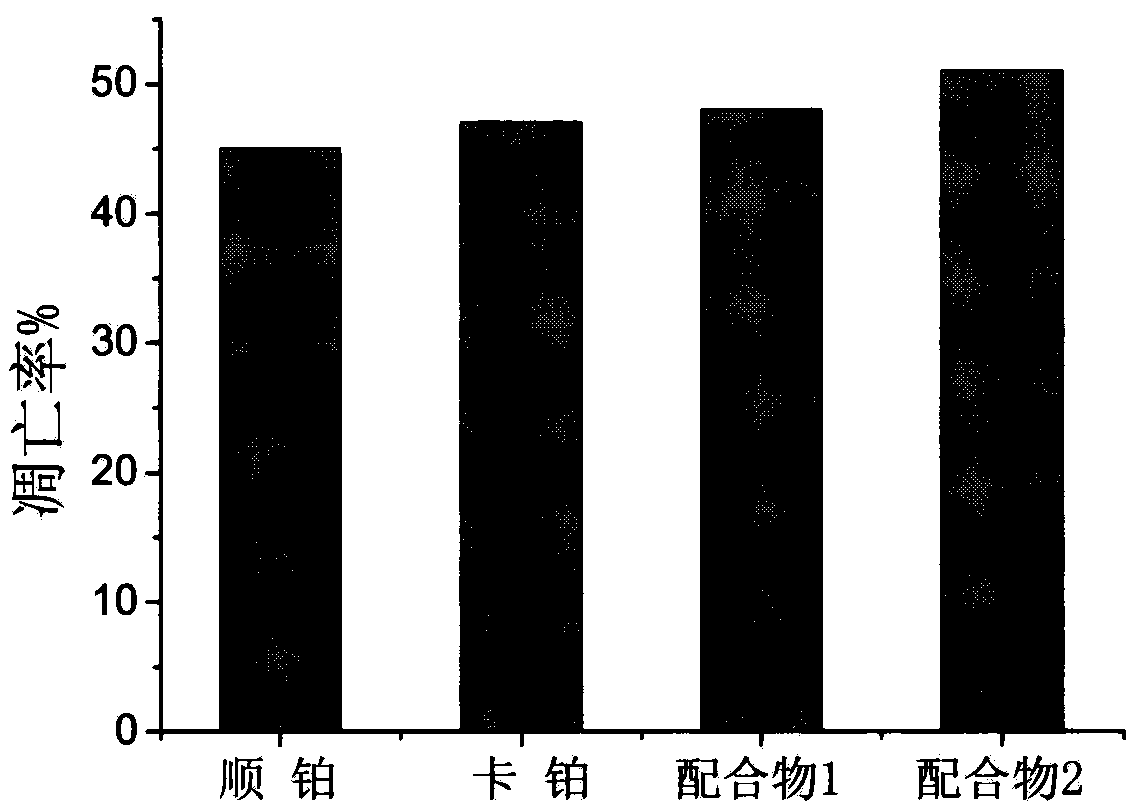
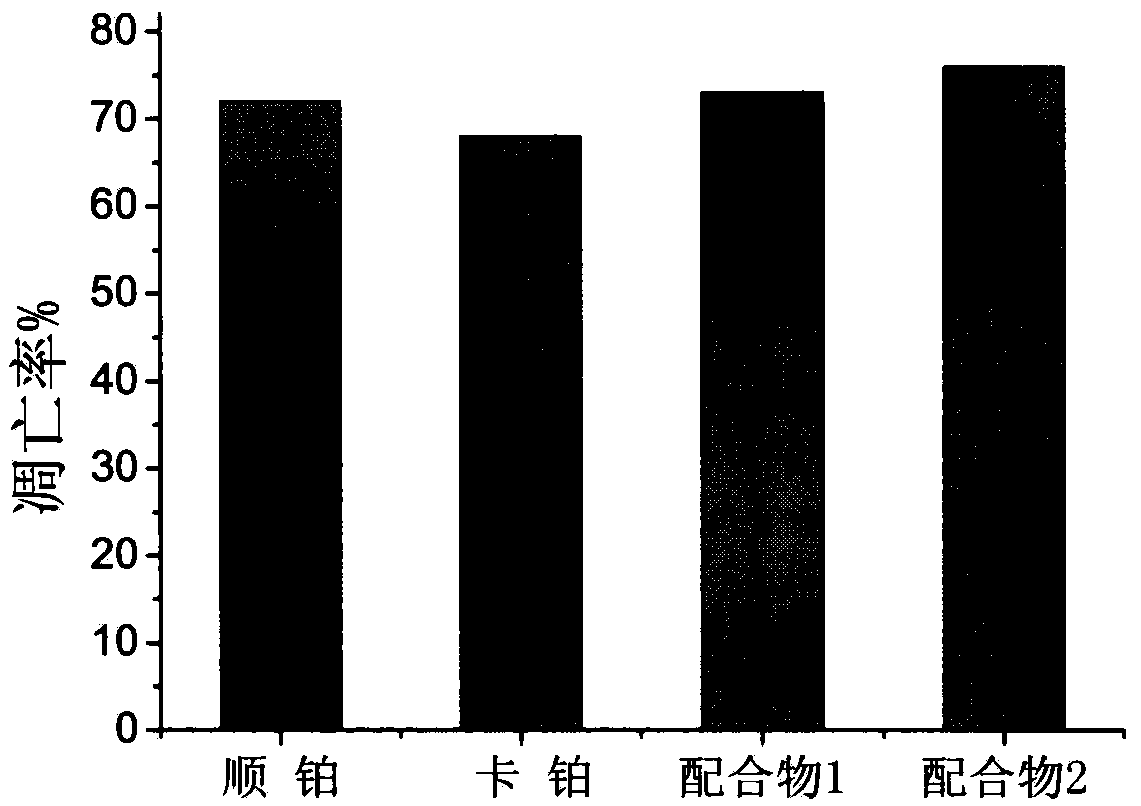
Preparation method and antitumor activity of ruthenium complexes containing benzothiazole
A technology of benzothiazole and ruthenium complexes, applied in the field of new ruthenium complexes, can solve the problems of large toxic and side effects, drug resistance of cancer cells, low water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
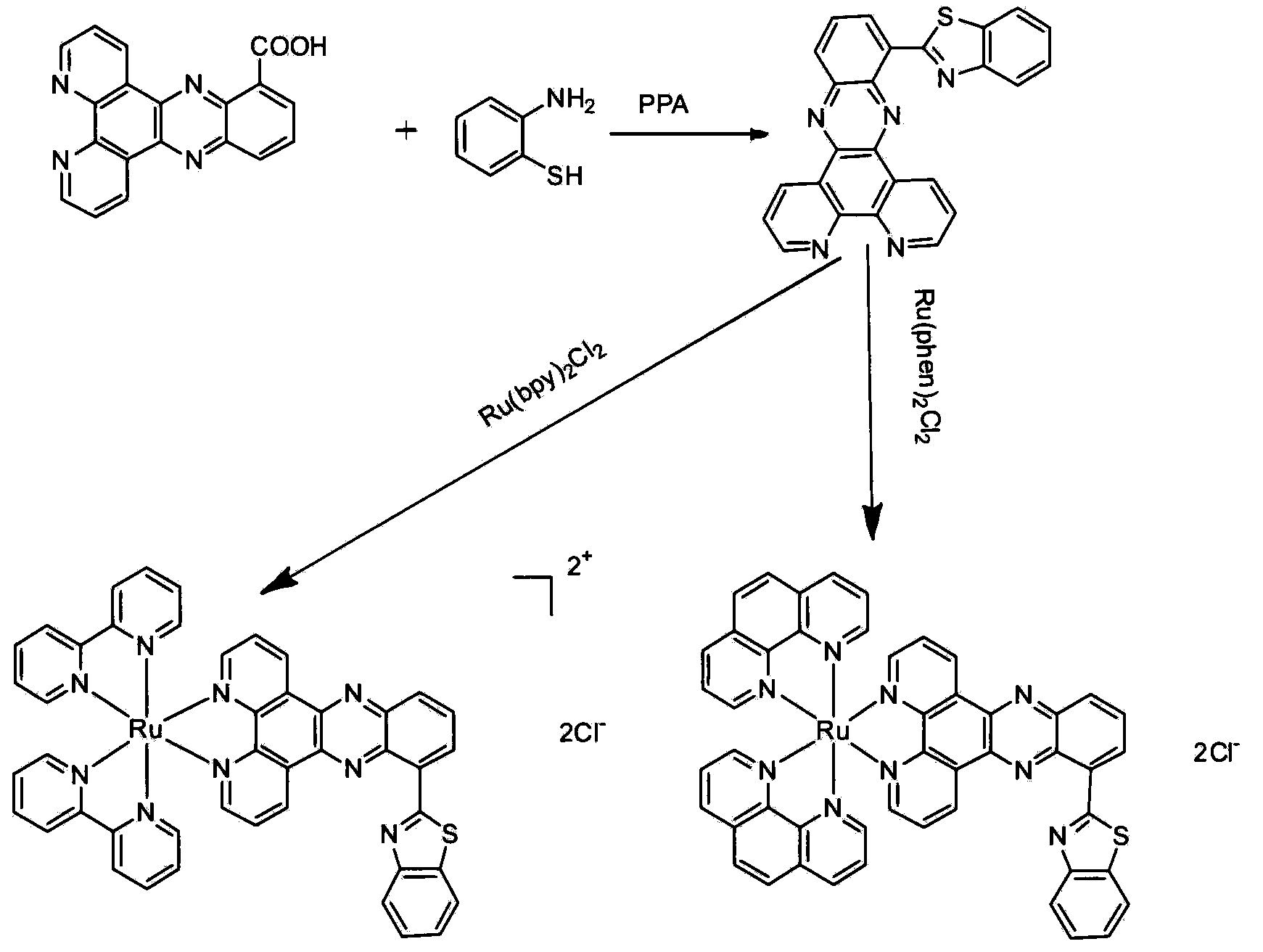
[0028] Example 1: Preparation of bipyridin[3,2-a:2',3'-c]phenazine-10-acid
[0029] Add 2.1 g (10 mmol) of 1,10-phenanthroline-5,6-dione (10 mmol) and 100 mL of ethanol into a 250 mL three-neck flask, and remove the air. Add 1.52g (10mmol) 2,3-diaminobenzoic acid under the protection of nitrogen, reflux at 80°C, stir the reaction, TLC thin layer chromatography to track the reaction progress, the reaction is completed after about 5h, after the reaction is stopped, cool, suction filter, and use After washing with dichloromethane and ethanol, 2.88 was obtained as a light yellow solid with a yield of 88.3%.
Embodiment 2
[0030] Example 2: Preparation of 2-(10-dipyridyl[3,2-a:2′,3′-c]phenazine)benzothiazole
[0031]In a 100mL three-necked flask, add 0.303g (0.929mmol) bipyridyl[3,2-a:2′,3′-c]phenazine-10-acid and an appropriate amount of polyphosphoric acid, nitrogen protection, and then pipette Pipette 1mL (0.929mmol) o-aminothiophenol, heat to 140°C with an oil bath, stir with a stirrer on, react for 20h, cool and pour into 100g of crushed ice, filter with suction, add 200mL of water to the filtrate , and the pH was adjusted to neutral with ammonia water, and suction filtered again, and the resulting solid was washed with water and ethanol. After drying, 0.192 g of a yellow-green solid was obtained, with a yield of 51.2%.
Embodiment 3
[0032] Embodiment 3: preparation [Ru(bpy) 2 BFDPP]Cl 2 and [Ru(phen) 2 BFDPP]Cl 2 :
[0033] Add 0.20mmol cis-Ru(bpy) successively to a 100mL three-neck flask 2 Cl 2 2H 2 O or cis-Ru(bpy) 2 Cl 2 2H 2 O and 0.2g (0.5mmol) 2-(10-dipyridyl[3,2-a:2′,3′-c]phenazine)benzothiazole, add 24mL ethanol, immerse the 6mL water glass tube into the liquid and blow with nitrogen gas Soak 20mIn to remove the dissolved oxygen in the solvent. The temperature was raised to 80°C, and the reaction was kept under reflux for 24 hours, and the reaction was stopped to obtain a red solution. The reaction solution was poured into 30mL deionized water and stirred for 5mIn. Suction filtration, discard the insoluble matter. The solvent in the filtrate was distilled off under reduced pressure, and the obtained solid was vacuum-dried. The dried solid was separated by column chromatography (neutral alumina column 200-300 mesh, eluent: acetonitrile / toluene=1 / 1). After the separation, the solvent w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com