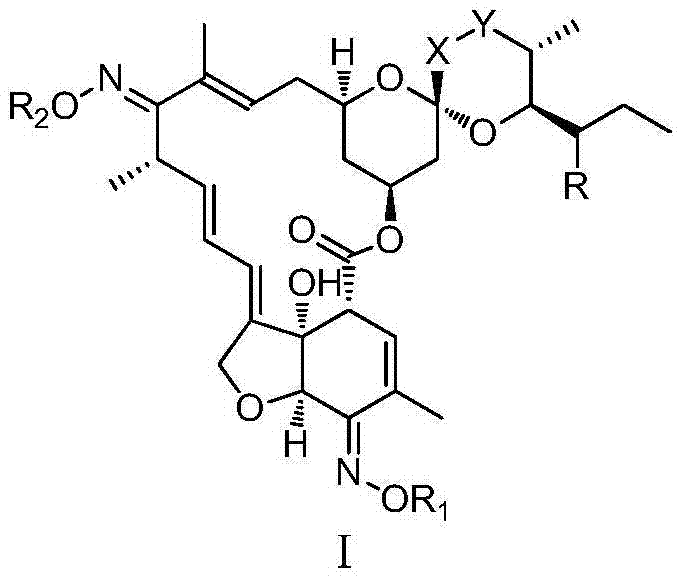
Milbemycin oxime compound and preparation method thereof
A technology of milbexime and compounds, which is applied in the field of milbexime compounds and their preparation, can solve the problems that no one has tried, and achieve the effects of simple operation, low synthesis cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
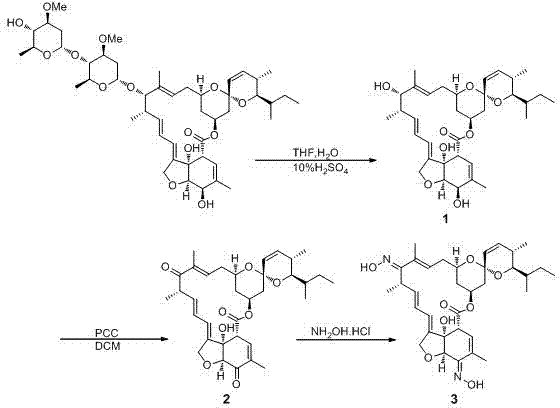
[0031] Embodiment 1: the preparation of compound 1
[0032] First, 2.35 mL of concentrated H 2 SO 4 and 2.31mL H 2 O mixed, added to 8.5mL of THF, mixed to form H 2 SO 4 / H 2 O / THF mixture. 222mg (0.25mmol) Abamectin B1a was added to a single-necked round bottom flask, and 10mL THF was added under argon protection to dissolve Abamectin B1a, then the flask was placed in an ice bath, and the prepared H 2 SO 4 / H 2 Slowly add the O / THF mixture into the THF solution of abamectin B1a. After the addition is complete, place it in an environment of 18°C for 24 hours. Monitor the progress of the reaction with thin-layer chromatography (TLC). After the reaction is complete, stir 15 mL of ice water was added to the reaction liquid under the conditions, and then extracted three times with dichloromethane (DCM), and the combined organic phase was washed with saturated 3 The solution and water were washed three times respectively, dried, spin-dried, and separated by a silica gel...
Embodiment 2
[0036] Embodiment 2: the preparation of compound 2
[0037] 25mg (0.043mmol) Compound 1 obtained in Example 1 was added to a 25mL single-necked round bottom flask, then 8mL of redistilled DCM was added for dissolution, and 27.6mg (3eq) of pyridinium chlorochromate (PCC ), mixed evenly to form a reaction solution, and stirred at room temperature for 6 h. The progress of the reaction was monitored by TLC. After the reaction was complete, 7 mL of water was added to the reaction solution, extracted three times with DCM, and the combined organic phase was washed three times with saturated brine / water (1 / 1, v / v), dried, spin-dried, and passed through silica gel. The target compound 2 was obtained by column separation with a yield of 91%.
[0038] The structure determination data of compound 2 are as follows:
[0039] 1 H NMR (400MHz, CDCl 3 ):δ6.56(s,1H),6.24(t,J=8.2Hz,1H),6.05(dd,J=14.6,11.3Hz,1H),5.94(d,J=11.3Hz,1H),5.84 –5.73(m,1H),5.55(dd,J=9.9,2.5Hz,1H),5.42(ddd,J=16.3,11....
Embodiment 3
[0041] Embodiment 3: the preparation of compound 3
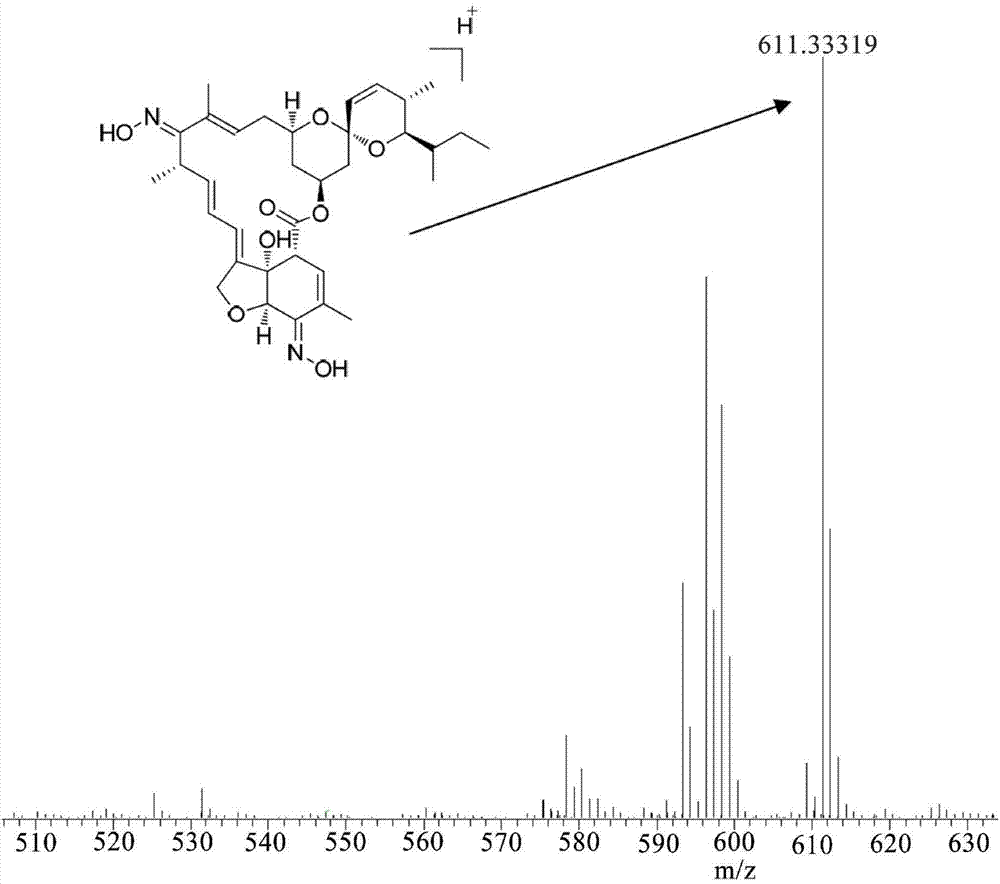
[0042] 99mg (0.17mmol) of compound 2 was added to a 10mL single-necked round bottom flask, then 18mL of isopropanol was added for dissolution, then 150mg of 4A molecular sieves were added, and the flask was then placed on an ice bath. Dissolve 244mg (20eq) of hydroxylamine hydrochloride in a small amount of methanol, slowly drop it into a round bottom flask, and react with magnetic stirring for 24h. The progress of the reaction was monitored by TLC. After the reaction was complete, it was extracted three times with anhydrous ether / water (1 / 1, v / v), and the combined organic phase was washed with water until the pH was close to neutral, dried, filtered and spin-dried, and passed through a silica gel column separation column The target compound 3 was obtained, which was the mirbe oxime compound, and the yield was 65%.
[0043] The structure determination data of compound 3 are as follows:
[0044] HRMS(m / z):calc.611.3332,foun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com