A kind of naringenin standard substance and its preparation and application
A naringenin, preparation-type technology, applied in the field of naringenin preparation, can solve the problems of high manufacturing cost, undisclosed naringenin standard substance preparation method, complexity, etc., and achieve large mass transfer coefficient and adjustable solubility , highly selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Embodiment 1. The preparation of naringenin
[0099] The method for preparing purified naringenin from citrus peel, its concrete steps are as follows:
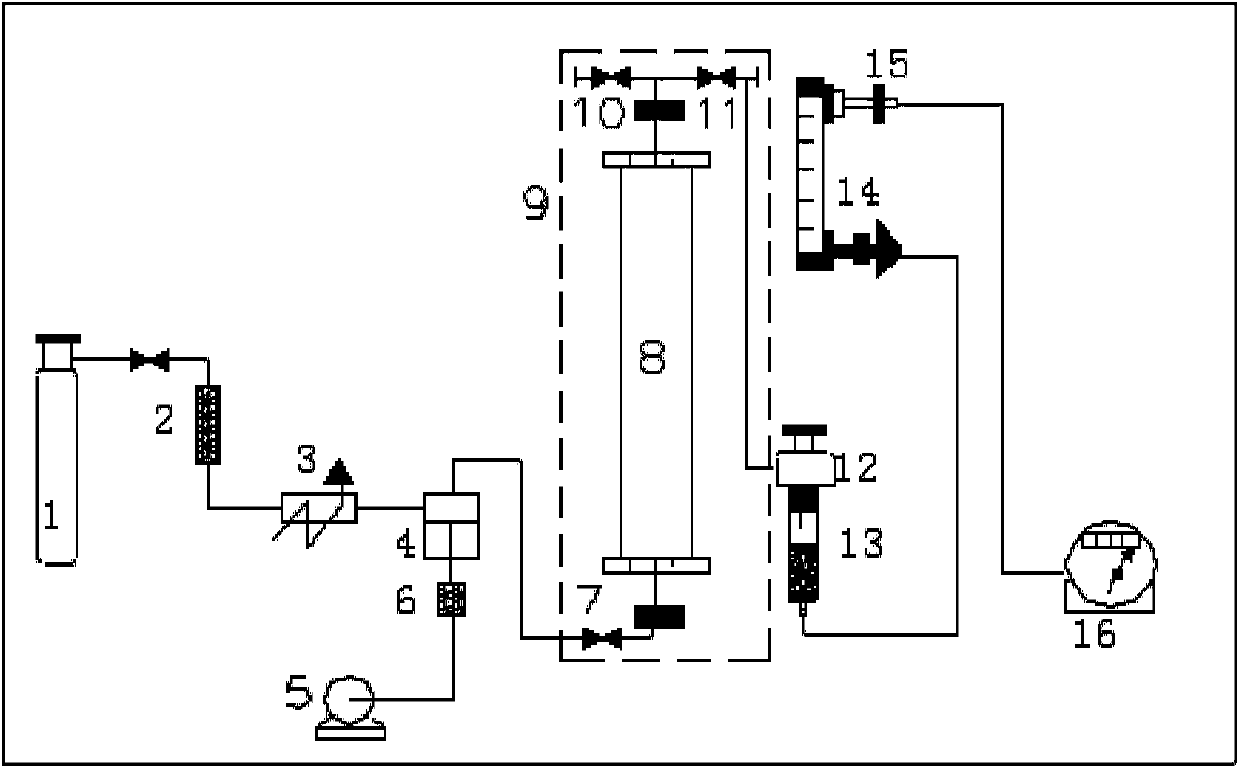
[0100] (1) Turn on the cooling heat exchanger to lower the temperature to about -5°C;
[0101] (2) Pack the pretreated experimental raw materials into the extraction kettle and seal it;
[0102] (3) Check and ensure that the back pressure valve is closed and the inlet valve of the high pressure pump is opened;
[0103] (4) Turn on the main power of the incubator 30 minutes before the operation and set the temperature of the extraction kettle and the separation kettle;
[0104] (5) Open the cylinder valve after the temperature meets the requirements;
[0105] (6) Turn on the high-pressure pump, observe the pressure on the computer screen, and confirm that the pressure meets the requirements;
[0106] (7) Before the pressure reaches the expected value, especially when it reaches about 10MPa, especially observe the val...
Embodiment 2-6
[0125] Except for the technical parameters listed in the table below, other preparation methods are the same as in Example 1 (see table 1 below for details).
[0126] Table 1
[0127]
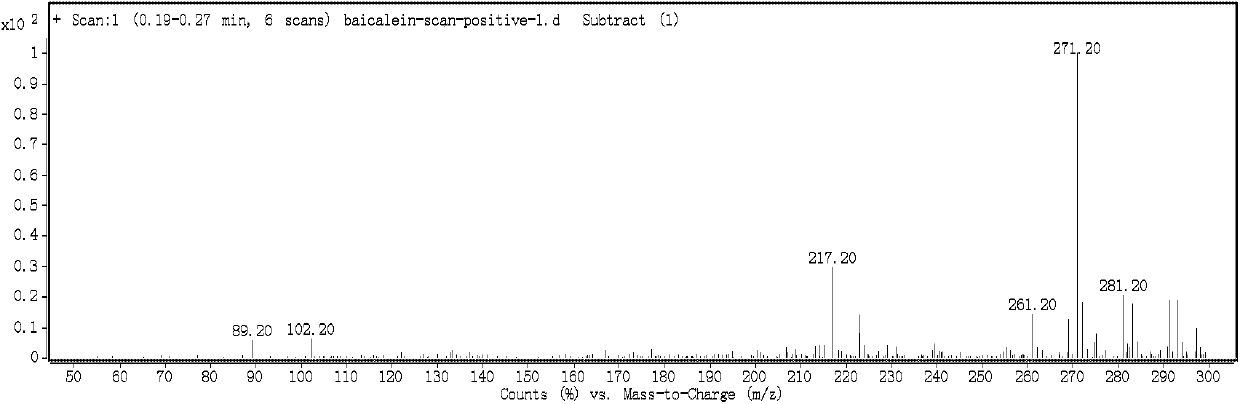
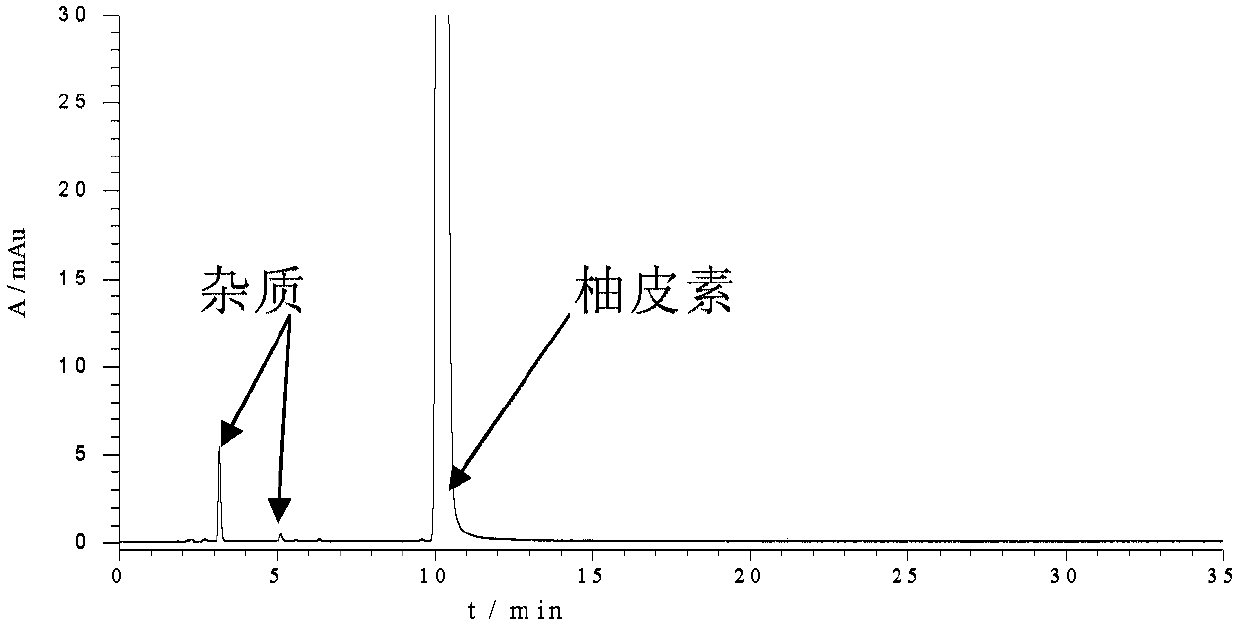
[0128] Similarly, the pure product of naringenin prepared in Example 2-6 was identified by HPLC-MS (ScanESI-MS) and HPLC fixed-value spectrum, which all showed that the naringenin was obtained by the extraction and separation method of Example 2-6. white.
Embodiment 7
[0129] The selection of the HPLC analysis condition of embodiment 7.naringenin standard substance
[0130] 7.1 Preparation of the test solution
[0131] Accurately weigh 10 mg of pure naringenin prepared in Example 1 and place it in a 5 mL volumetric flask, add an appropriate amount of water to dissolve and dilute to the mark to obtain a naringenin solution with a concentration of 2 mg / mL, which is used to determine the chromatographic separation conditions and fixed value use.
[0132] 7.2 Determination of chromatographic conditions
[0133] 7.2.1 Selection of mobile phase
[0134] The mobile phase composed of different ratios of water:methanol was investigated respectively, and 15%:85% (water:methanol) was selected as the mobile phase.
[0135] 7.2.2 Selection of chromatographic column:
[0136] The C18 chromatographic column was selected for testing, and the final chromatographic conditions were as follows:
[0137] Chromatographic column: Agilent ZORBAXExtend-C1821.2*...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com