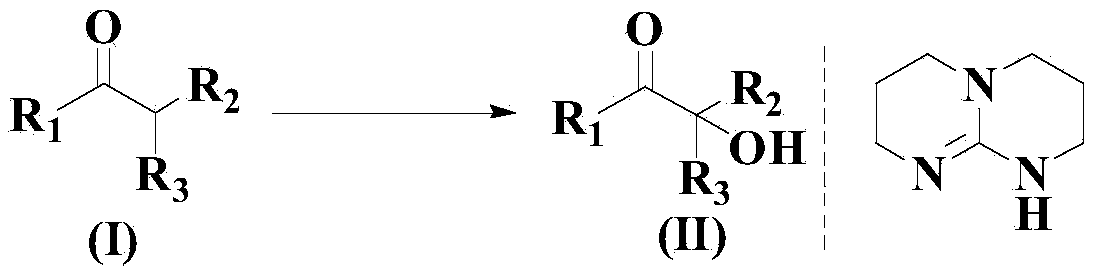
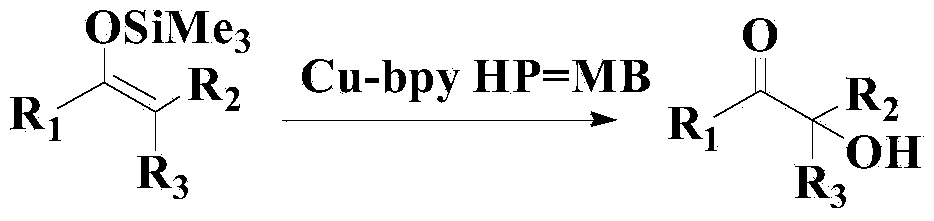Novel synthesis method for three-stage alpha-hydroxyl carbonyl compound
A synthesis method and compound technology, applied in the field of organic chemical synthesis, can solve the problems of increased production cost, insufficient environmental protection, unsuitable for large-scale production, etc., achieving the effects of abundant sources and overcoming low reaction yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034]
[0035] Add 1 mol formula (I) compound, 2.05 mol sodium carbonate and 1.9 mol isopropyltriphenylphosphine bromide successively in the reactor at room temperature, and keep stirring under oxygen atmosphere, then add 8L DMSO and mass ratio thereto 1:0.7:0.5 of N,N-diisopropylethylamine (DIPEA), SmI 2 and a mixture additive of ferrocene (total mass is 2000g), the mixture is continuously stirred under an oxygen atmosphere at room temperature, and reacted for a total of 18h. After the reaction is completed, ethyl acetate is added for dilution, followed by washing with salt water and extraction with ethyl acetate. The organic phase was dried over anhydrous magnesium sulfate, filtered and concentrated in vacuo, and the residue was purified by column chromatography to obtain the compound of formula (II) with a yield of 97.5% and a purity of 98.7% (HPLC).
[0036] 1 H NMR (400MHz, CDCl 3)δ=7.46-7.41(m,2H),7.23(t,J=7.6Hz,1H),7.15(t,J=9.2Hz,1H),3.06(brs,1H),1.53(s,6H);
[0...
Embodiment 2
[0039]
[0040] Add 1 mol formula (I) compound, 2.15 mol sodium carbonate and 1.8 mol isopropyltriphenylphosphine bromide successively in the reactor at room temperature, and keep stirring under oxygen atmosphere, then add 7L DMSO and mass ratio thereto 1:0.7:0.5 of N,N-diisopropylethylamine (DIPEA), SmI 2 and a mixture additive of ferrocene (total mass is 4000g), the mixture is continuously stirred under an oxygen atmosphere at room temperature, and reacted for a total of 15h. After the reaction is completed, ethyl acetate is added for dilution, followed by washing with salt water and extraction with ethyl acetate. The organic phase was dried over anhydrous magnesium sulfate, filtered and concentrated in vacuo, and the residue was purified by column chromatography to obtain the compound of formula (II) with a yield of 96.9% and a purity of 98.6% (HPLC).
[0041] 1 H NMR (400MHz, CDCl 3 )δ=9.26(s,1H),8.73(d,J=4.8Hz,1H),8.30(d,J=8.0Hz,1H),7.45-7.42(m,1H),3.57(brs,1H), 1.6...
Embodiment 3
[0044]
[0045] Add 1 mol formula (I) compound, 2.1 mol sodium carbonate and 1.9 mol isopropyltriphenylphosphine bromide successively in the reactor at room temperature, and keep stirring under oxygen atmosphere, then add 9L DMSO and mass ratio thereto 1:0.7:0.5 of N,N-diisopropylethylamine (DIPEA), SmI 2 and a mixture additive of ferrocene (total mass is 5000g), the mixture is continuously stirred under an oxygen atmosphere at room temperature, and reacted for a total of 17h. After the reaction is completed, ethyl acetate is added for dilution, followed by washing with salt water and extraction with ethyl acetate. The organic phase was dried over anhydrous magnesium sulfate, filtered and concentrated in vacuo, and the residue was purified by column chromatography to obtain the compound of formula (II) with a yield of 96.3% and a purity of 98.4% (HPLC).
[0046] 1 H NMR (400MHz, CDCl 3 )δ=7.91(d,J=3.6Hz,1H),7.67(d,J=5.2Hz,1H),7.14(t,J=5.2Hz,1H),3.42(brs,1H),1.63(s, 6H); ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com