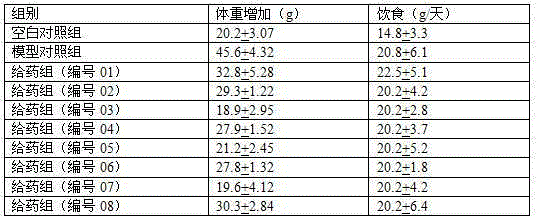
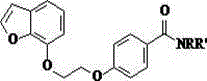
Benzofuran compounds and their medicinal uses
A technology for benzofuran and compound, which is applied in the field of benzofuran compounds and their medicinal uses, can solve problems such as difficulty in controlling intestinal symptoms, and achieve the effects of inhibiting fat absorption, preventing catalytic decomposition, and being highly selective.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
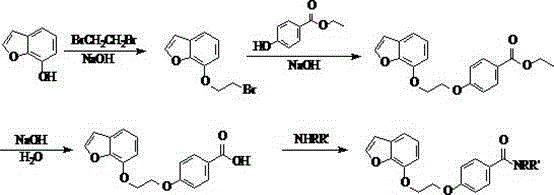
[0056] Example 1: Preparation of 4-[2-(benzofuran-7 base)ethoxy]benzamide (Compound No. 01)
[0057] 1. Preparation of 7-(2-bromoethyl)benzofuran
[0058]Add 18.00g (0.13mol) of 7-hydroxybenzofuran, 22.68g (0.14mol) of 1,2-dibromoethane, 6.00g (0.04mol) of potassium iodide, and 49.63g (0.36mol) of potassium carbonate into the reaction flask. Acetone was used as solvent and heated to reflux for 3.5h. Suction filtration, rotary evaporation to remove acetone, add an appropriate amount of petroleum ether to dissolve, extract once with 5% NaOH aqueous solution, collect the organic layer and dry it with anhydrous magnesium sulfate. Filtrate, and remove petroleum ether by rotary evaporation to obtain a light yellow oil, add a small amount of petroleum ether to freeze, and a white solid precipitates out. Filter and dry to obtain 22.36g of white solid, yield 69.13%, mp: 72.7-74.5°C. GC-MS m / z: 239.9; 1 H-NMR (CDCl 3 )δ(ppm): 1.98(2H,m), 2.20(2H,m), 2.71(2H,t), 3.72(2H,t), 4.06(2H,...
Embodiment 2
[0065] Example 2: Preparation of N,N-dimethyl-4-[2-(benzofuran-7yl)ethoxy]benzamide (Compound No. 02)
[0066] According to the method of example 1, by 4-[3-(chroman-7-oxygen) propoxyl group] benzoic acid 0.66g (0.002mol), dimethylamine 0.09g (0.002mol), EDC0.42g (0.0022 mol), HOBt0.03g (0.0002mol), triethylamine 1.00g (0.01mol) to give 0.51g of white solid, yield 78.46%, mp: 118.4-119.2℃. LC-MS m / z: 326.1 ([M+H] + ),348.1([M+Na] + ),364.1([M+K] + ); 1 H-NMR (CDCl 3 )δ (ppm): 3.10 (6H, s), 4.28 (2H, m), 4.32 (2H, m), 6.52 (2H, d, J =8.4Hz),6.82(2H,d, J =7.5Hzt),6.98(2H,d, J =8.7Hz),7.08(2H,m),7.20(1H,d, J =7.8Hz),7.45(1H,d, J =8.4Hz),7.66(2H,d, J =8.7Hz); IR(KBr)υ / cm -1 : 3318, 2719, 1642, 1153, 1046, 1153, 1122, 1008, 831, 612.
Embodiment 3
[0067] Example 3: Preparation of N,N-diethyl-4-[2-(benzofuran-7yl)ethoxy]benzamide (Compound No. 03)
[0068] According to the method of example 1, by 4-[3-(chroman-7-oxygen) propoxyl group] benzoic acid 0.66g (0.002mol), diethylamine 0.15g (0.002mol), EDC0.42g (0.0022 mol), HOBt0.03g (0.0002mol), triethylamine 1.00g (0.01mol) to give 0.53g of white solid, yield 74.65%, mp: 129.3-131.2℃. LC-MS m / z: 354.2 ([M+H] + ),376.1([M+Na] + ),392.2([M+K] + ); 1 H-NMR (CDCl 3 )δ(ppm): 1.02(6H,t, J =2.4Hz),2.98(4H,q, J =2.4Hz),4.27(2H,m),4.34(2H,m),6.54(2H,d, J =8.4Hz),6.85(2H,d, J =7.5Hzt),7.02(2H,d, J =8.7Hz),7.12(2H,m),7.21(1H,d, J =7.8Hz),7.55(1H,d, J =8.4Hz),7.69(2H,d, J =8.7Hz); IR(KBr)υ / cm -1 : 3326, 2765, 1621, 1415, 1354, 1115, 1098, 1096, 1060, 732.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com