Double-labeling time-resolved fluorescence immunoassay method and kit for HIV (human immunodeficiency virus) antibody and HIV-1p24 antigen
A time-resolved fluorescence, hiv-1p24 technology, applied in the direction of analytical materials, biological material analysis, measurement devices, etc., can solve the problems of shortening the HIV detection window period, low HIV detection sensitivity, poor stability, etc., and achieve simple detection methods and high sensitivity High and stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of kit
[0033] The kit of this embodiment 1 includes: a solid phase carrier coated with HIV antigen and HIV-1p24 monoclonal antibody at the same time, calibrator, biotin-labeled HIV-1p24 monoclonal antibody, HIV antigen labeled with lanthanide 1, Lanthanide 2-labeled Streptavidin, Assay Buffer, Wash Concentrate, and Enhancement Solution. Its specific preparation method is as follows:
[0034] ① Preparation of antigen and antibody solid-phase carrier: HIV recombinant antigen (including HIV gp120, gp41, gp36 epitopes, which can detect HIV-1 type, HIV-2 type, HIV-10 subgroup) and HIV-1p24 monoclonal antibody Coating buffer (you can use pH9.6, 50mmol / L carbonic acid buffer; pH4.5, 20mmol / L phosphate buffer; pH7.8, 50mmol / L Tris-HCl buffer or pH4.5, 50mmol / L citrate buffer, etc.) were diluted to 0.1, 0.5, 1, 2, 5, 10μg / mL concentration as coating solution, and HIV antigen coating solution and HIV- 100 μl / well of 1p24 antibody coating solutio...
Embodiment 2
[0045] Example 2: Detection and Analysis of HIV Antibody and HIV-1p24 Antigen
[0046] ① Reagent preparation
[0047] Antigen and antibody solid-phase carrier: Equilibrate the required amount of antigen and antibody solid-phase carrier prepared in Example 1 to room temperature (20-25°C). The remaining antigens and antibody solid phase carriers were placed in ziplock bags in time to be sealed and stored at 2-8°C.
[0048] Washing liquid: Mix 40ml of concentrated washing liquid and 960ml of purified water in a clean container in Example 1, and use it as a working washing liquid for subsequent use.
[0049] Marker working solution: the Eu prepared in Example 1 3+ Labeled HIV recombinant antigen and Sm 3+ Labeled streptavidin and experimental buffer were added into the same clean disposable container at a volume ratio of 1:1:20 and mixed well, prepared within 30 minutes before use, and used up in the current experiment.
[0050] Biotin-labeled antibody working solution: Add bi...
Embodiment 3
[0054] Embodiment 3: detection result analysis
[0055] (1) Detection performance of HIV antibody
[0056] ① Conformity rate of negative reference products: 20 national negative reference products for HIV antibody detection, no more than 2 positive reactions (≥18 / 20).
[0057] ②Conformity rate of positive reference products: 18 national HIV1 antibody positive reference products were detected, all of which were positive reactions (18 / 18), and the detection fluorescence values of samples P11 and P12 were P12≥P11; 2 HIV2 antibody positive reference products , were positive (2 / 2).
[0058] ③Minimum detection limit: 6 copies of the national minimum detection limit reference product were tested, no less than 3 copies were positive and the matrix serum S1 was negative.
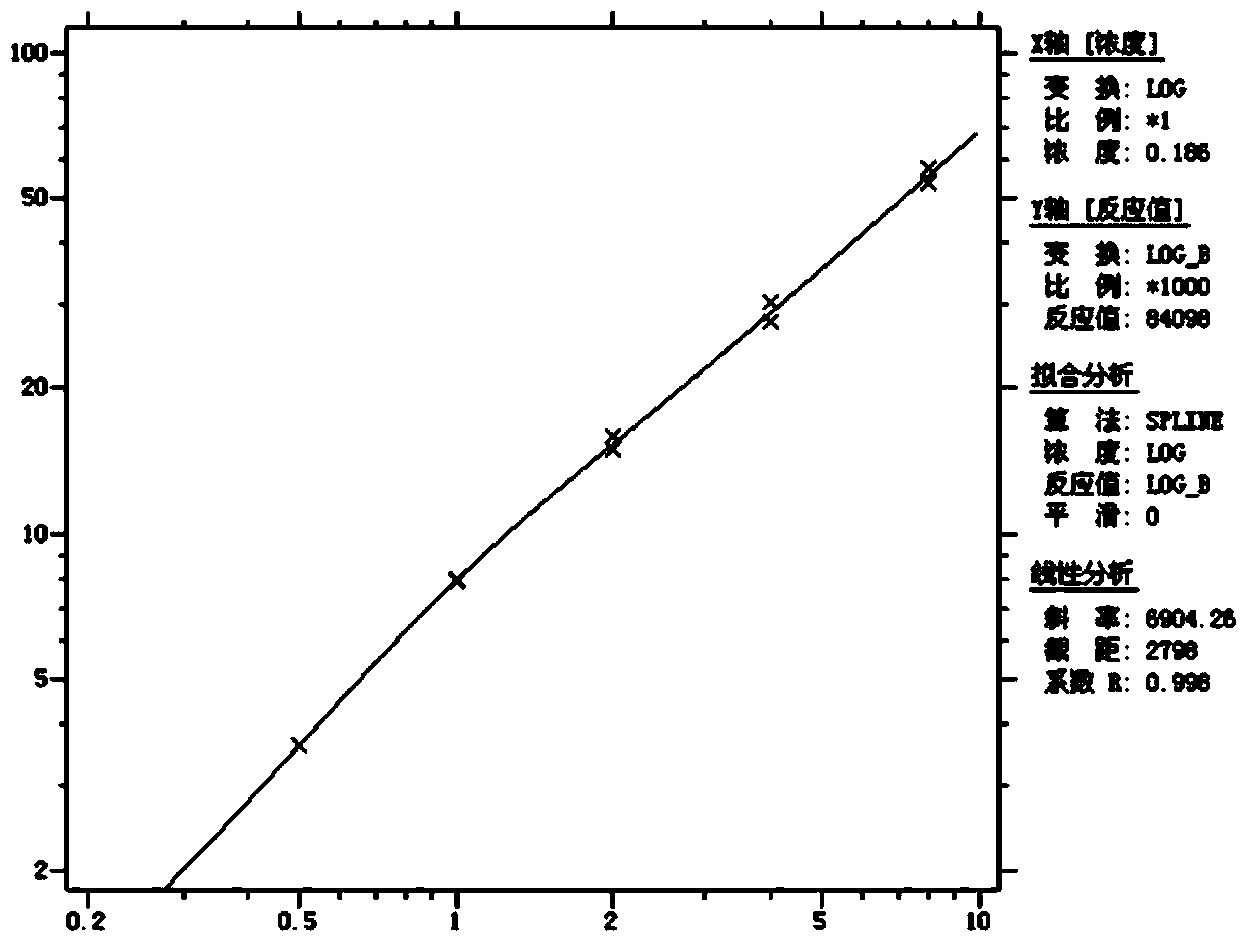
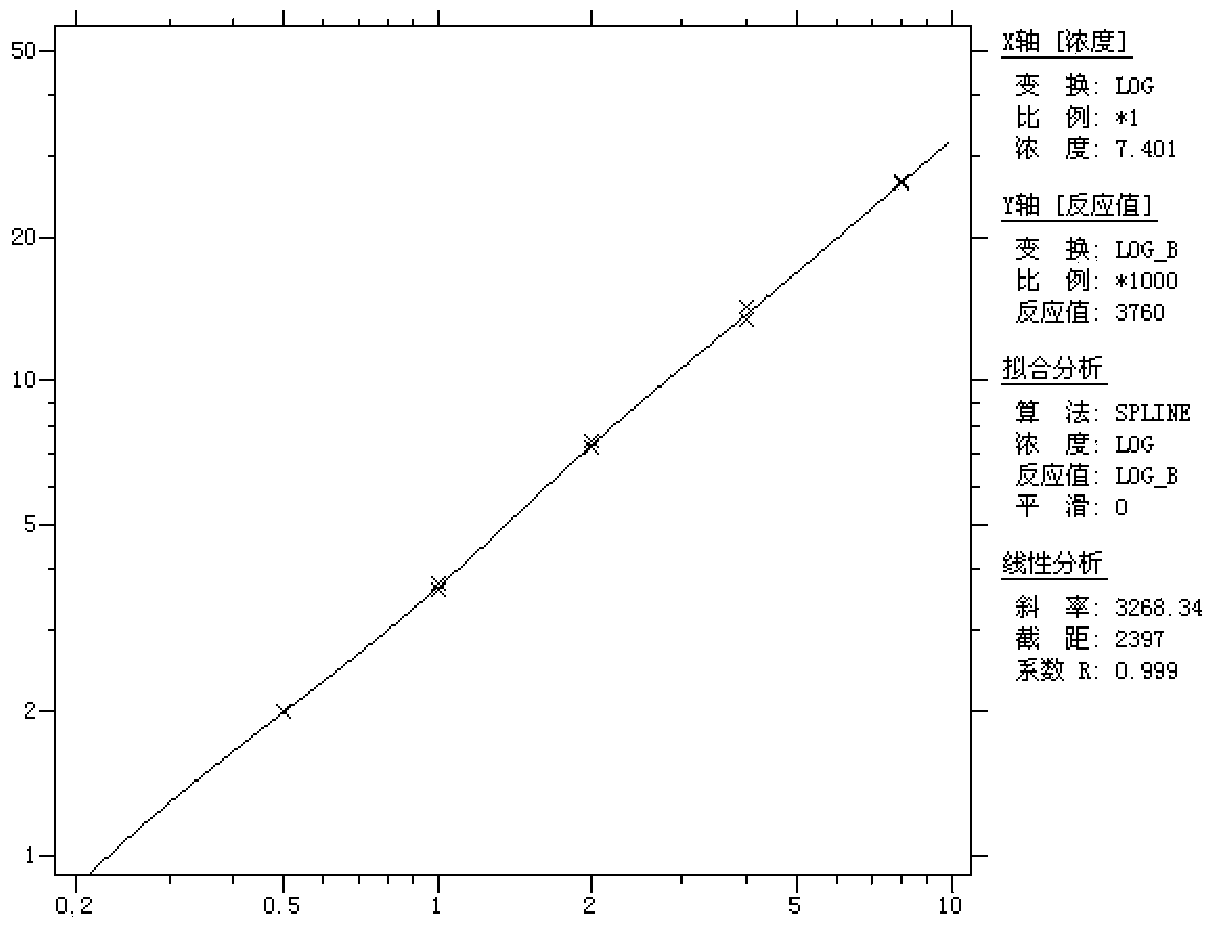
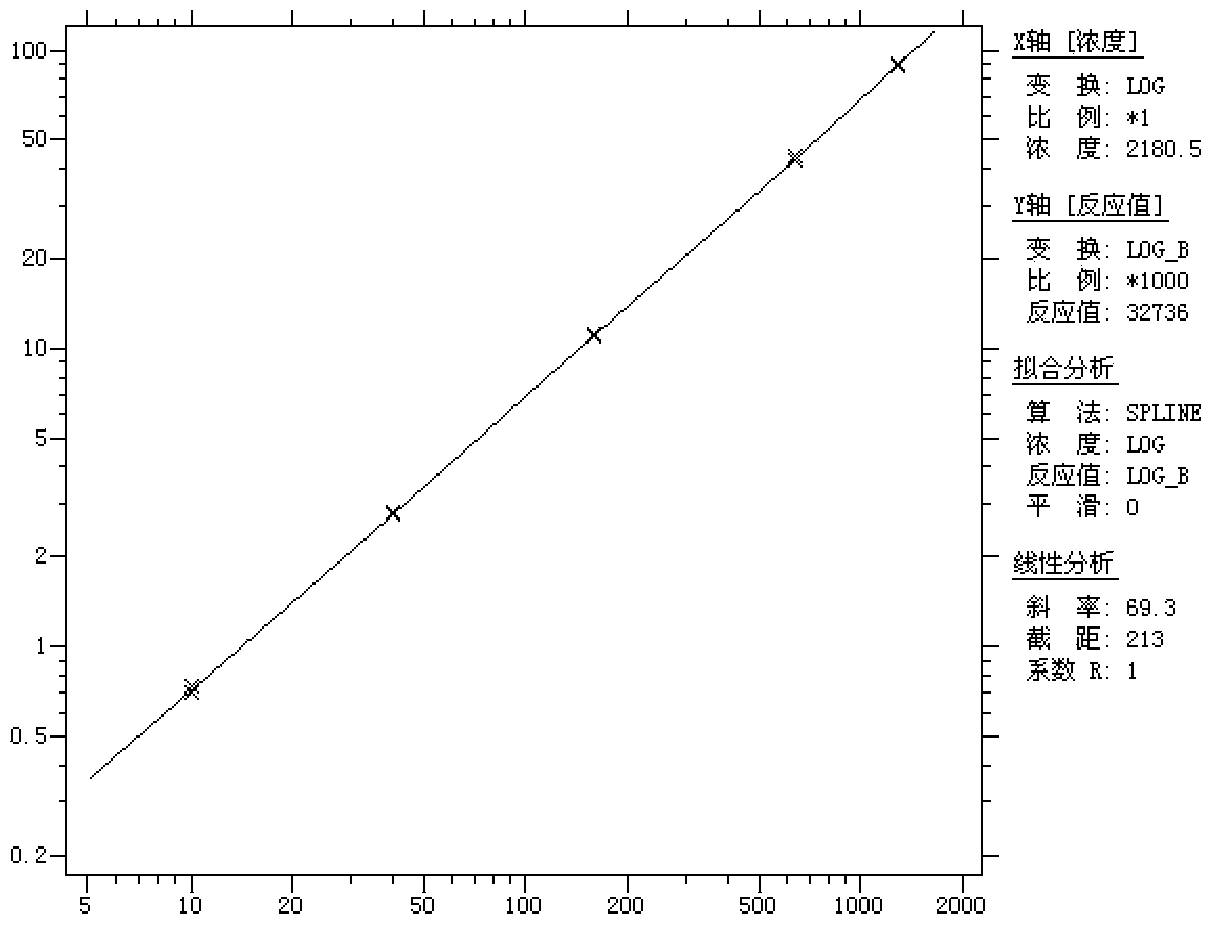
[0059] ④Linearity: After testing the reference products of the enterprise, after statistical analysis of the measured values of 5 samples from L1 to L5, the linear correlation coefficient r between the measured...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com