Application of beta hydroxybutyric acid in treatment of Parkinson disease
A technology for β-hydroxybutyric acid and Parkinson's disease, applied to nervous system diseases, anhydride/acid/halide active ingredients, drug combinations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: β-Hydroxybutyrate reduces ROS in dopamine neurons in a rotenone PD cell model.
[0025] Day1: will be cultured at 25cm 2 The SH-SY5Y cells in the culture flask were made into a cell suspension, spread in a 6-well plate, about 1×10 cells per well 6 .
[0026] Day2: The cells were about 30-40% confluent, and β-hydroxybutyric acid was administered at a final concentration of 1, 2.5, and 5 mM.
[0027] Day3: Add rotenone after 24 hours, the final concentration is 0.5 μM.
[0028] Day4: After 24 hours, aspirate the medium, wash with PBS, add 1 ml of DCFH-DA fluorescent probe diluted 1:1000 with serum-free medium, incubate at 37°C for 30 min, wash the cells 3 times with serum-free medium, trypsin After digestion, centrifuge, resuspend in 1 ml PBS, and detect the fluorescence of DCF by flow cytometry. The results are shown in Table 1.
[0029] Table 1. Effect of β-hydroxybutyric acid on ROS production of dopamine neurons in rotenone PD cell model (n=6)
[003...
Embodiment 2
[0032] Example 2: Beta hydroxybutyrate reduces MPP + PD cell model dopamine neuron ROS.
[0033] Day1: will be cultured at 25cm 2 The SH-SY5Y cells in the culture flask were made into a cell suspension, spread in a 6-well plate, about 1×10 cells per well 6 .
[0034] Day2: The cells were about 30-40% confluent, and β-hydroxybutyric acid was administered at a final concentration of 1, 2.5, and 5 mM.
[0035] Day3: Join MPP after 24 hours + , with a final concentration of 1 mM.
[0036] Day4: After 24 hours, aspirate the medium, wash with PBS, add 1 ml of DCFH-DA fluorescent probe diluted 1:1000 with serum-free medium, incubate at 37°C for 30 min, wash the cells 3 times with serum-free medium, trypsin After digestion, centrifuge, resuspend in 1 ml PBS, and detect the fluorescence of DCF by flow cytometry. The results are shown in Table 2.
[0037] Table 2. Effect of β-hydroxybutyrate on MPP + PD cell model dopamine neurons produce ROS effects (n=6)
[0038] ...
Embodiment 3
[0040] Example 3: Effects of β-hydroxybutyrate on apoptosis of dopamine neurons in a rotenone PD cell model.
[0041] Apoptosis——Hoechst 33342 staining detection
[0042] Day1: Spread the dopamine neuron cell line SH-SY5Y on a 24-well plate.
[0043] Day2: The cells were about 30-40% confluent, and β-hydroxybutyric acid was administered at a final concentration of 1, 2.5, and 5 mM.
[0044] Day3: Add rotenone after 24 hours, the final concentration is 0.5 μM.
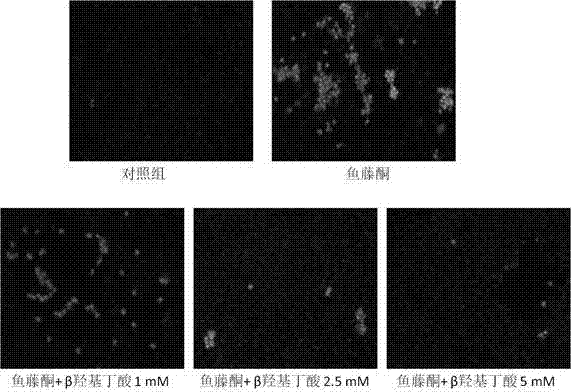
[0045] Day4: After 24 hours, Hoechst 33342 staining was performed, and cell apoptosis was detected by fluorescence microscopy.
[0046] The results showed that the rotenone PD cell model had obvious apoptosis, while pre-administration of β-hydroxybutyrate reduced the apoptosis induced by rotenone (see figure 1 ).
[0047] Apoptosis——PI Flow Cytometry
[0048] Day1: will be cultured at 25cm 2 The SH-SY5Y cells in the culture flask were made into a cell suspension, spread in a 6-well plate, about 1×10 cells per we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com