Method for preparing 9β, 10-α-dehydroprogesterone ketal by dual-wavelength microfluidic technology and dual-wavelength microfluidic photochemical reactor
A photochemical reaction and chemical reactor technology, applied in the field of organic photochemical synthesis, can solve the problems of low mass transfer and heat transfer efficiency, insufficient light, uneven photoreaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
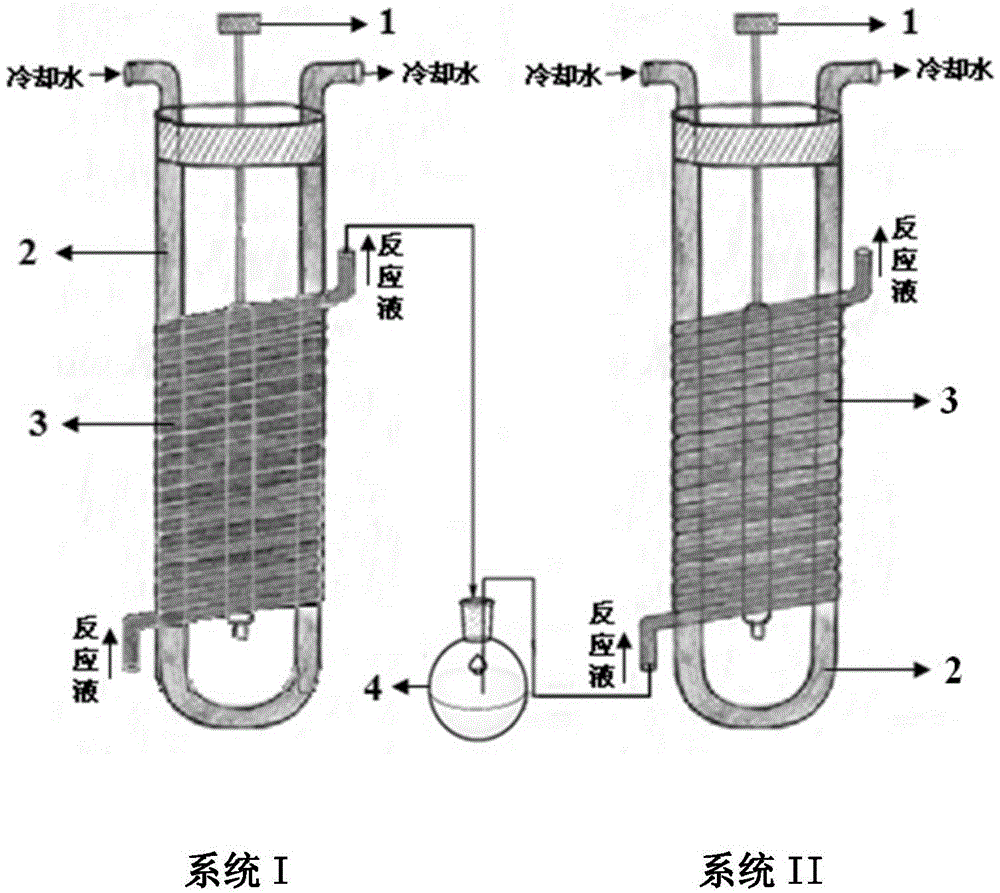
[0032] Such as figure 1 As shown, the dual-wavelength microflow photochemical reactor for the synthesis of 9β, 10α-dehydroprogesterone ketal consists of system I and system II, and both system I and system II are an independent microflow photochemical reactor, Except for the different filter wavelengths of the cold trap, the other structures are exactly the same; they are composed of a light source 1, a cold trap 2 and a microfluidic tube 3 respectively.
[0033] The cold trap is a U-shaped interlayer container with cooling water inlet and outlet, the micro-flow tube made of quartz or high borosilicate glass is wound on the outer wall of the cold trap, and the light source It is placed in the inner cavity of the cold trap and located at the place where the micro-flow tube is wound.
[0034] The liquid outlet of the micro-fluid tube in the system I communicates with a storage tank 4 through a pipeline, and the storage tank communicates with the liquid inlet of the micro-fluid ...
Embodiment 2
[0049] The 9β, 10α-dehydroprogesterone ketal was synthesized using the dual-wavelength microflow photochemical reactor used in Example 1 for the synthesis of 9β, 10α-dehydroprogesterone ketal.
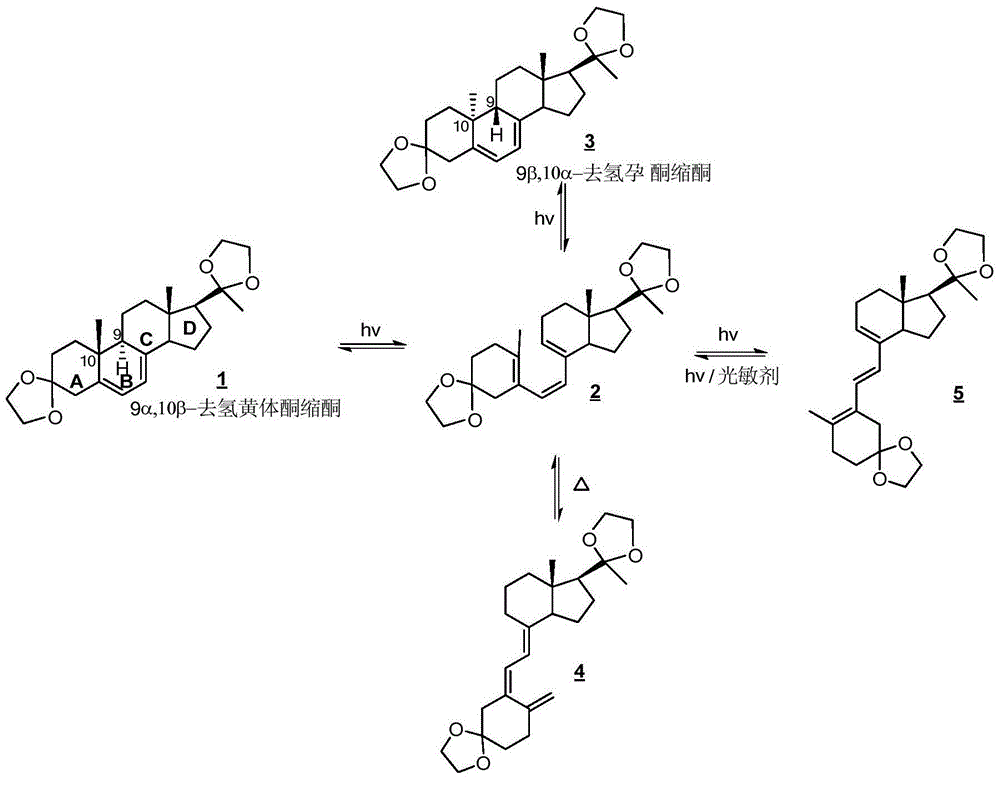
[0050] (1) 9α,10β-Dehydroprogesterone Ketal B Ring Photochemical Bond Breaking Reaction
[0051] At 30°C, add 22.5 g of 9α,10β-dehydroprogesterone ketal dissolved in 1500 mL of tetrahydrofuran into a 2000 mL round bottom flask, and then add 20 mg of 2,6-di-tert-butyl-p-methoxyphenol and 8 mg of collidine, stirred and mixed evenly, and prepared into a photochemical reaction solution;
[0052] Feed nitrogen into the prepared photochemical reaction solution, open the 1000W high-pressure mercury lamp placed in the inner cavity of the cold trap that constitutes the dual-wavelength microflow photochemical reactor system I after 30 minutes, and open the cooling water valve of the system I, While cooling the cold trap, use a peristaltic pump to pump the photochemical reaction liquid into the ...
Embodiment 3
[0060] The 9β, 10α-dehydroprogesterone ketal was synthesized using the dual-wavelength microflow photochemical reactor used in Example 1 for the synthesis of 9β, 10α-dehydroprogesterone ketal.
[0061] (1) 9α,10β-Dehydroprogesterone Ketal B Ring Photochemical Bond Breaking Reaction
[0062] At 28°C, add 10 g of 9α,10β-dehydroprogesterone ketal dissolved in 2000 mL of dioxane to a 2500 mL round bottom flask, and then add 12 mg of 2,6-di-tert-butyl-p-methyl Phenol and 4 mg of pyridine were stirred and mixed uniformly to prepare a photochemical reaction solution;
[0063] Feed nitrogen into the prepared photochemical reaction solution, open the 500W high-pressure mercury lamp placed in the inner chamber of the cold trap that constitutes the dual-wavelength microflow photochemical reactor system I after 30 minutes, and open the cooling water valve of the system I. While cooling the cold trap, use a peristaltic pump to pump the photochemical reaction liquid into the micro-flow tub...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com