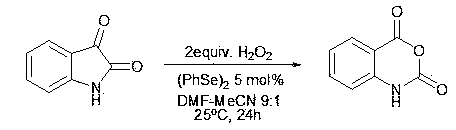Synthesis method of isatoic anhydride and derivative thereof
A synthetic method, the technology of isatoic anhydride, applied in the direction of organic chemistry, etc., can solve the problems of serious equipment corrosion, not a synthetic method, etc., and achieve the effect of low equipment corrosion and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016] Add 1.47 g of isatin (10 mmol), 0.16 g of diphenyldiselenide (0.5 mmol) into a round bottom flask. Under stirring, keep the temperature of the mixed system at 25°C, and slowly add 25 ml of DMF-acetonitrile mixed solution (DMF volume content 10%) dissolved in 20 mmol mass concentration of 30% hydrogen peroxide solution dropwise. The mixture was stirred and reacted for 24 hours at a system temperature of 25°C.
[0017] After the reaction was completed, 30 ml of 5% ferrous chloride aqueous solution was added, extracted with ethyl acetate (50 ml 3 times), the organic layers were combined, dried with anhydrous sodium sulfate, evaporated to dryness, and the residue was separated by column chromatography to obtain indigo Red acid anhydride 1.39 g (yield 85%).
Embodiment 2
[0019] Other organoselenium catalysts were used to catalyze the reaction, and other conditions were the same as in Example 1. The experimental results are shown in Table 1.
[0020] Table 1 The reaction yields catalyzed by different organoselenium catalysts
[0021] serial number catalyst Yield(%) 1 diphenyl diselenide 85 (Example 1) 2 4,4'-Dimethoxydiphenyl diselenide 83 3 4,4'-Difluorodiphenyldiselenide 77 4 Dicyclohexyl diselenide 72 5 Benzene selenite 66 6 Phenylselenium bromide 70 7 diphenyl selenide 32 8 Phenylcyclohexylselenide 66
[0022] It can be seen from Table 1 that diphenyl diselenide is the best catalyst (Example 1).
Embodiment 3
[0024] Different amounts of diphenyldiselenide were used as catalysts, and other conditions were the same as in Example 1. The experimental results are shown in Table 2.
[0025] Table 2 Test of catalyst dosage effect:
[0026] serial number The amount of catalyst relative to isatin (mol%) Yield(%) 1 0.1 66 2 0.5 72 3 1 75 4 5 85 (Example 1) 5 10 83 6 15 78 7 20 73
[0027] It can be seen from Table 2 that the molar ratio of the catalyst relative to the amount of isatin is 1:20, and the effect is the best (Example 1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com