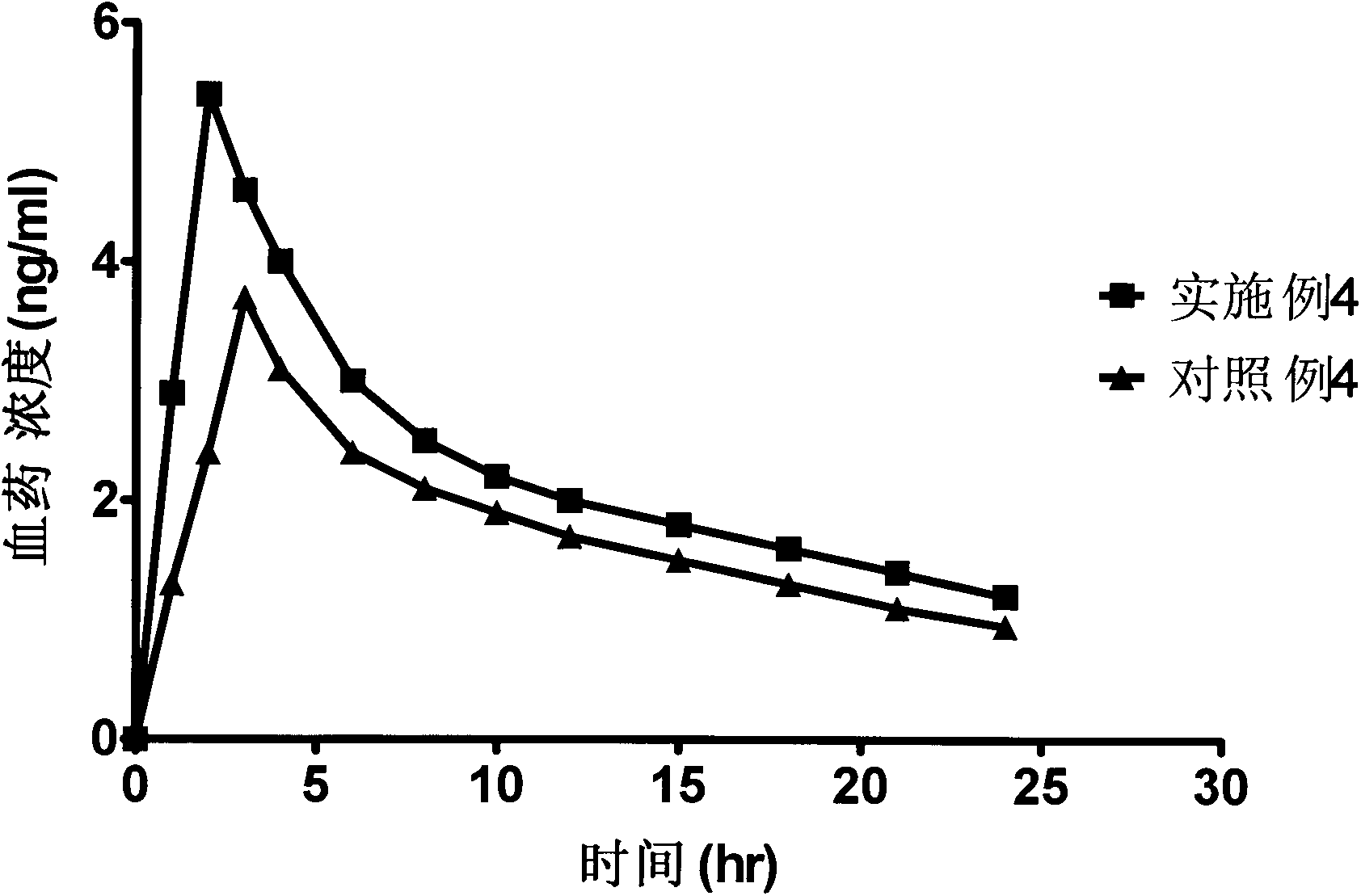
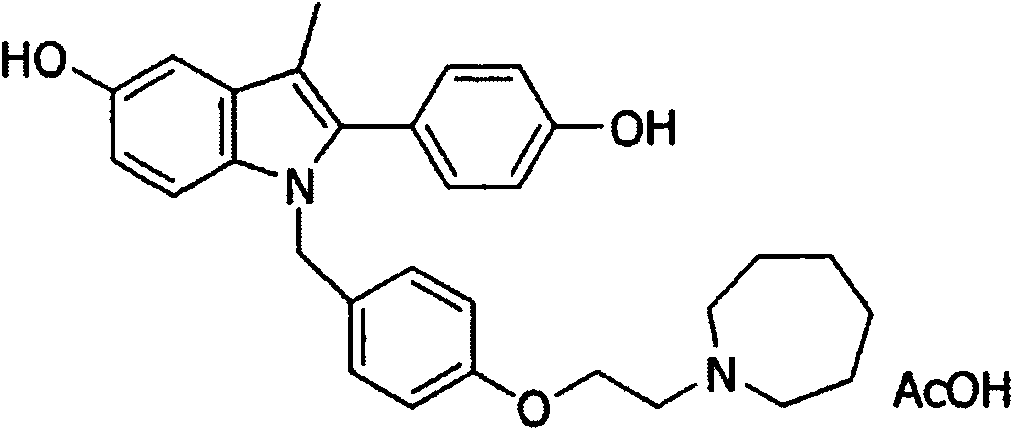
Bazedoxifene acetate composition with excellent property
A technology of bazedoxifene acetate and composition, applied in the field of bazedoxifene acetate composition with excellent performance, can solve problems such as affecting chemical stability, and achieve the effects of fast drug release speed and high chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Prescription: Dosage per 1000 capsules:
[0033] Bazedoxifene Acetate 4.52g
[0034] Vitamin E TPGS 15.0g
[0035] PEG 6000 30.48g
[0036] Specifications: main drug content 4.52mg / grain, pill weight 50mg / grain.
[0037] Process: Crush and finely grind bazedoxifene acetate raw materials to more than 150 mesh; weigh vitamin E TPGS and PEG 6000 according to the prescription; heat and melt vitamin E TPGS, add bazedoxifene acetate and mix well, then add PEG 6000 Mix well to form a suspension; heat the above eutectic solution at 80-90°C; transfer the above solution into a dripping device for dripping, control the temperature of the solution at 80-90°C, and drop it into simethicone oil at room temperature , take out and drain the silicone oil, wipe off the silicone oil attached to the surface of the dripping pill with medicinal gauze, and that's it.
Embodiment 2
[0039] Prescription: Dosage per 1000 capsules:
[0040] Bazedoxifene Acetate 2.26g
[0041] Vitamin E TPGS 2.74g
[0042] PEG 8000 45g
[0043] Specifications: main drug content 2.26mg / grain, pill weight 50mg / grain.
[0044] Process: Crush and finely grind the bazedoxifene acetate raw material to more than 150 meshes; weigh vitamin E TPGS and PEG 8000 according to the prescription; heat and melt the vitamin E TPGS, add bazedoxifene acetate and mix well, then add PEG 8000 Mix well to form a suspension; heat the above eutectic solution at 80-85°C; transfer the above solution into a dripping device for dripping, control the temperature of the solution at 80-85°C, and drop it into simethicone oil at room temperature , take out and drain the silicone oil, wipe off the silicone oil attached to the surface of the dripping pill with medicinal gauze, and that's it.
Embodiment 3
[0046] Prescription: Dosage per 1000 tablets:
[0047]
[0048]
[0049] Specifications: main drug content 22.6mg / tablet, weight 500mg / tablet.
[0050] Process: 1) Pulverize the bazedoxifene acetate raw material and grind it to a fineness of more than 150 mesh; heat and melt the vitamin E TPGS, add bazedoxifene acetate and mix well, then add PEG 6000 and mix well to form a suspension eutectic liquid; the above-mentioned eutectic liquid is cooled to room temperature and crushed to 60-80 mesh; 2), take lactose monohydrate, microcrystalline cellulose, pregelatinized starch, sodium starch glycolate and pass through 80 mesh sieve, add in process 1 The pulverized product, colloidal silicon dioxide, and magnesium stearate are mixed evenly and compressed into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com