Transdermal delivery preparation in three-dimensional netty spatial configuration of agomelatine and preparation method thereof
A three-dimensional network and three-dimensional configuration technology, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. The shortcomings of bioavailability have not been basically improved, the dissolution testing method has not been disclosed, and the dissolution results are still under discussion, so as to achieve the effect of fast transdermal absorption, avoiding first-pass effect, and reducing the frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] prescription:
[0057]
[0058] The nano silica is selected from the VK-SP15E nano silica produced by Xuancheng Jingrui New Material Co., Ltd., with an average particle size of 15nm;
[0059] The dispersant is propylene glycol;
[0060] The pressure-sensitive adhesive is acrylic pressure-sensitive adhesive 87-4098;
[0061] The transdermal penetration enhancer is Azone (Azone)
[0062] (1) Dissolve the drug in absolute ethanol with twice the saturated solubility of the drug, disperse the nano-silica material in the dispersant, mix the above two solutions, sonicate for 0.5 hours, and soak for another 4 hours to obtain a uniformly dispersed three-dimensional network Drug-carrying system with three-dimensional configuration;
[0063] (2) Add a transdermal penetration enhancer to the pressure-sensitive adhesive, add ethyl acetate to adjust the viscosity of the glue to 1500cp at 25°C, and stir at 2000rpm for 2 hours to obtain the glue for use;
[0064] (3) Add the product of step (1) t...
Embodiment 2
[0072] The agomelatine transdermal patch was prepared by the same method as in Example 1. The composition of the prescription is as follows:
[0073]
[0074]
[0075] The nano-silica is selected from the VK-SP20E nano-silica produced by Xuancheng Jingrui New Material Co., Ltd., with an average particle size of 20nm, dispersing agent is PEG200; pressure-sensitive adhesive is silicone pressure-sensitive adhesive 4032; permeation promotion The agent is isopropyl myristate (IPM)
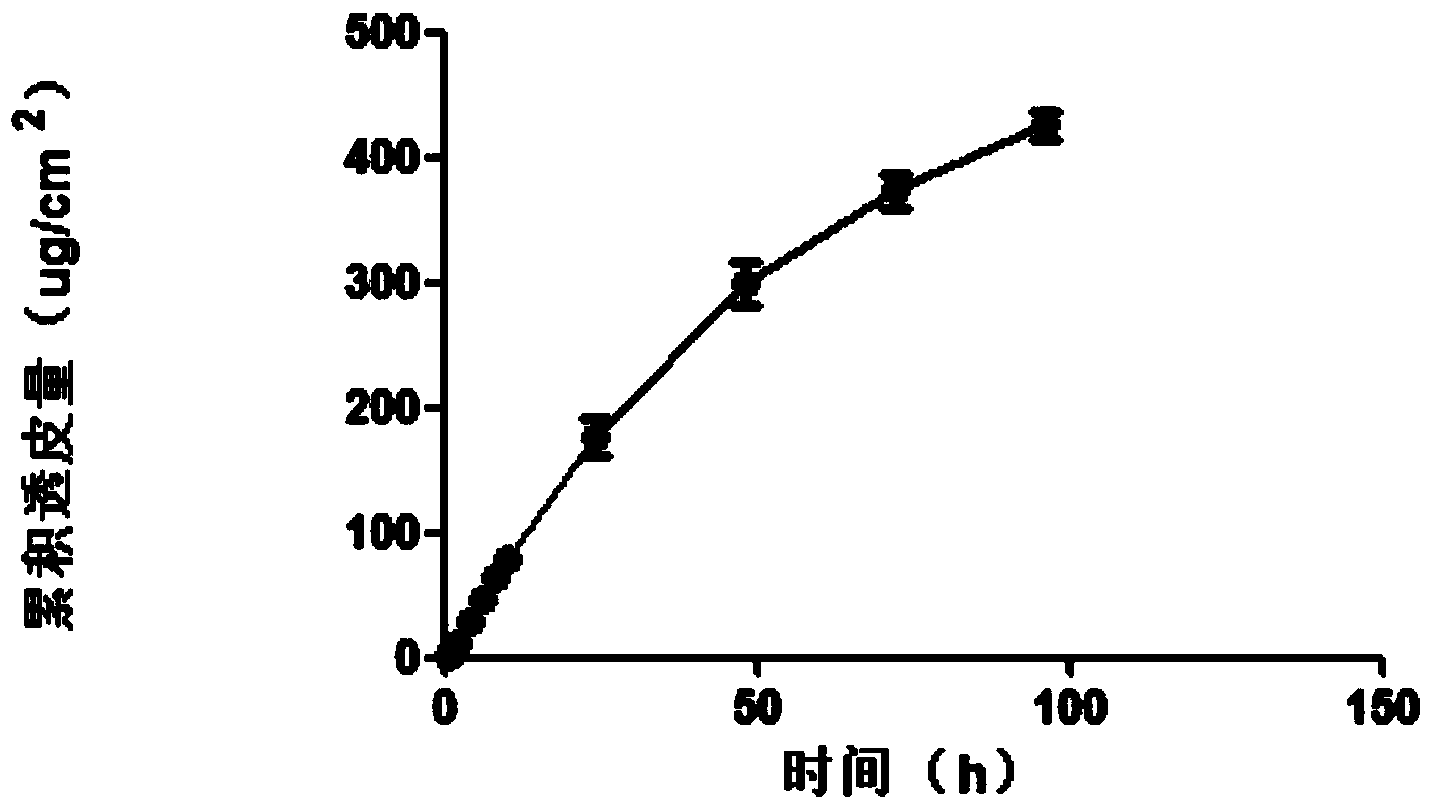
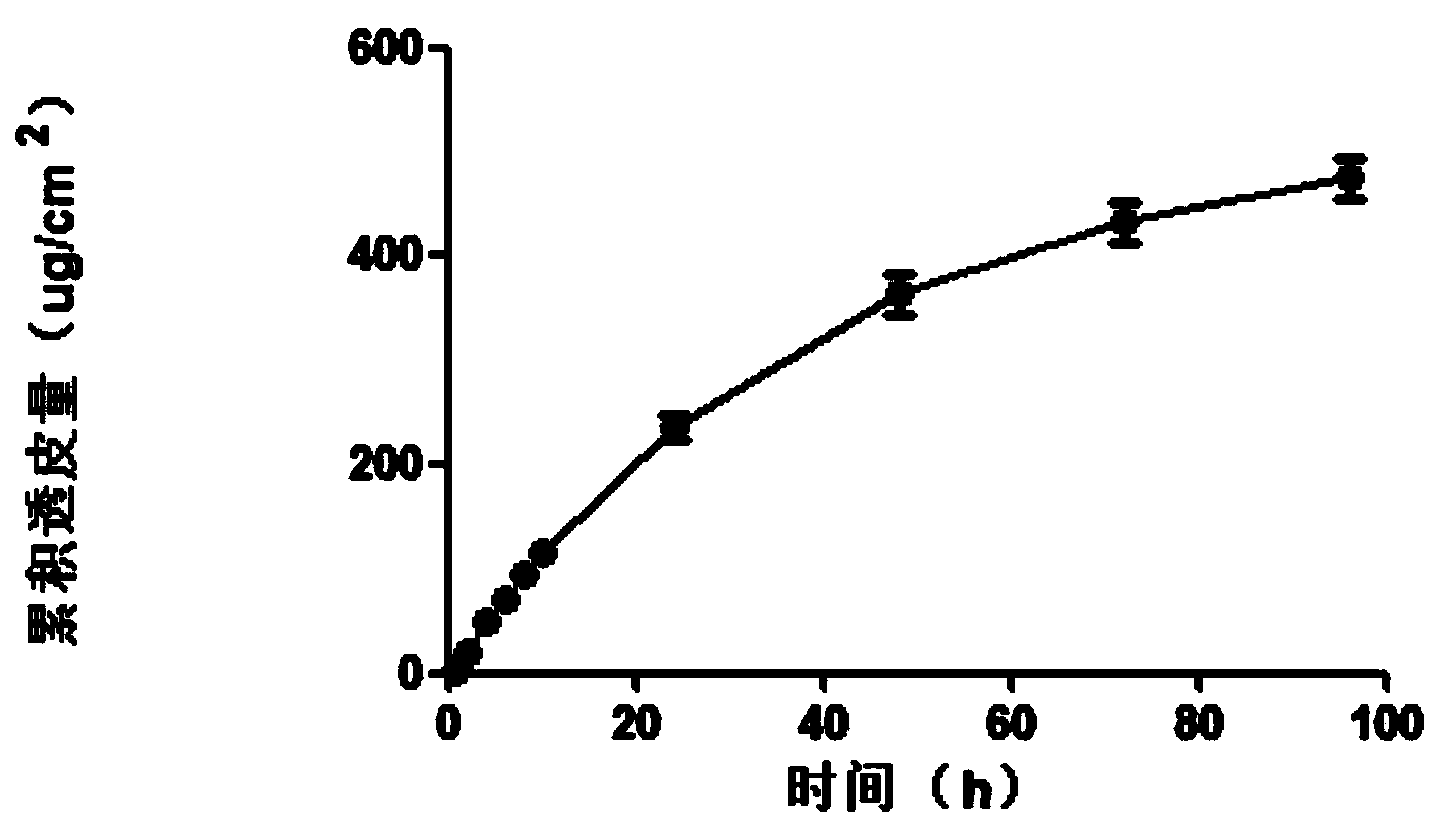
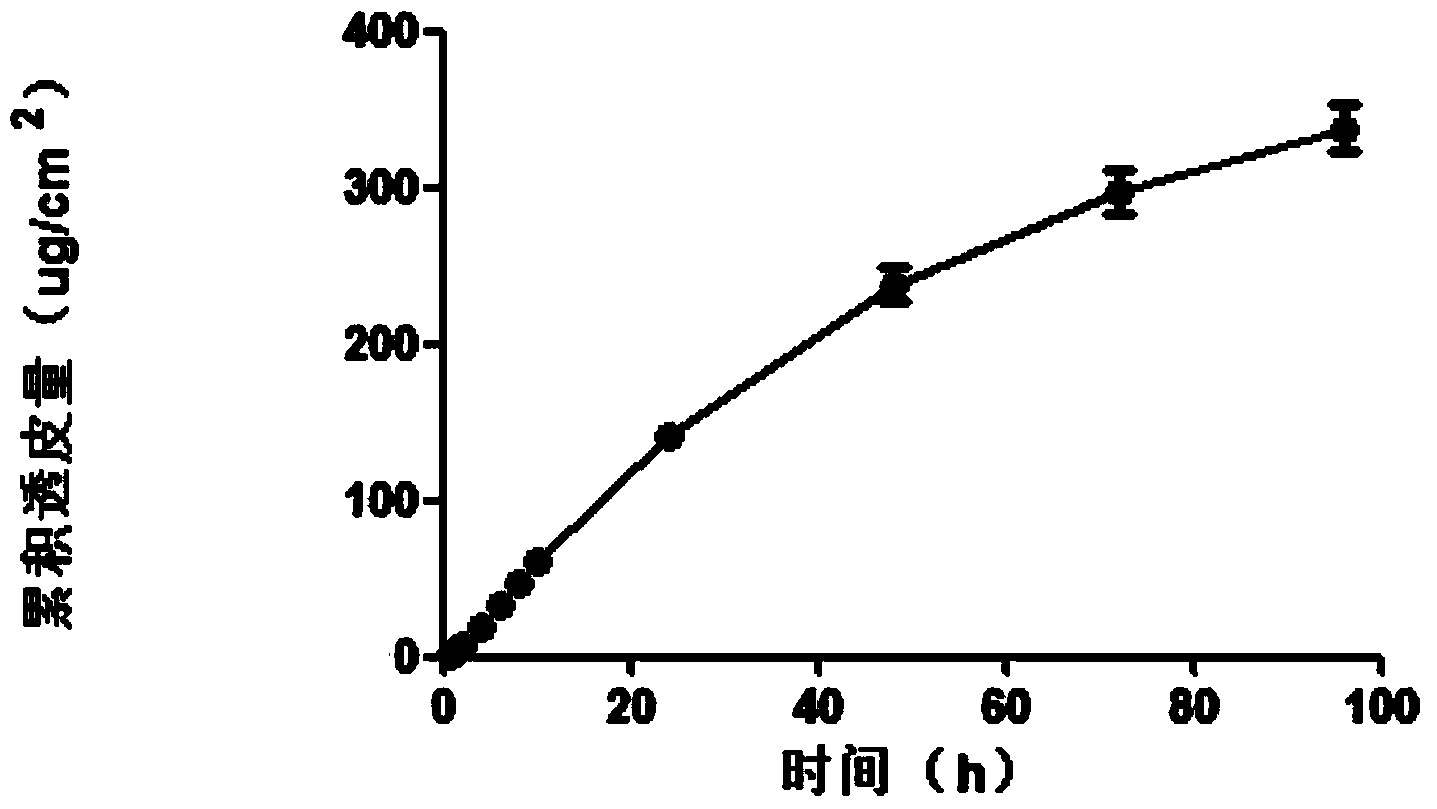
[0076] The agomelatine transdermal patch was prepared by the method of Example 1, and the in vitro transdermal experiment was performed using the same method. The average rate of agomelatine transdermal patch through the skin of nude mice was 4.95ug / cm 2 .h. Uniform rapid release of the drug for 96h, indicating that the in vitro transdermal process conforms to the zero-order release process at 96h, and the controlled release is good. See the cumulative transdermal transport rate of agomelatine transdermal...
Embodiment 3
[0078] The agomelatine transdermal patch was prepared by the same method as in Example 1. The composition of the prescription is as follows:
[0079]
[0080] The nano-silica is selected from the VK-SP25E nano-silica produced by Xuancheng Jingrui New Materials Co., Ltd., with an average particle size of 25nm, and the dispersant is propylene glycol; the pressure-sensitive adhesive is acrylic pressure-sensitive adhesive 87-2287; transdermal The penetration enhancer is glycerol triacetate. The agomelatine transdermal patch was prepared by the method of Example 1, and the in vitro transdermal test was carried out using the same method, then the agomelatine transdermal patch penetrated naked The average velocity of mouse skin is 3.52ug / cm 2 .h. Uniform rapid release of the drug for 96h, indicating that the in vitro transdermal process conforms to the zero-order release process at 96h, and the controlled release is good. See the cumulative transdermal transport rate of agomelatine tra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com